Bencyclane
Appearance
(Redirected from C19H31NO)

| |

| |
| Names | |
|---|---|
| IUPAC name
3-[(1-benzylcycloheptyl)oxy]-N,N-dimethylpropan-1-amine
| |
| Identifiers | |
3D model (JSmol)
|
|
| ChEMBL | |
| ChemSpider | |
| ECHA InfoCard | 100.016.861 |
PubChem CID
|
|
| UNII | |
CompTox Dashboard (EPA)
|
|
| |
| |
| Properties | |
| C19H31NO | |
| Molar mass | 289.45554 |
| Pharmacology | |
| C04AX11 (WHO) | |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
| |
Bencyclane is an antispasmodic, vasodilator, and platelet aggregation inhibitor.[1]
Synthesis
[edit]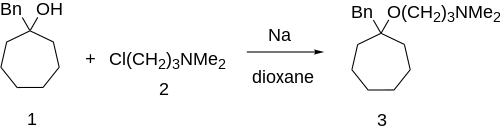
Grignard addition of benzylmagnesiumbromide to suberone would give 1-benzylcycloheptanol [4006-73-9] (1'). Williamson ether synthesis with 3-dimethylaminopropylchloride [109-54-6] (2) completed the synthesis of bencyclane (3).
See also
[edit]References
[edit]- ^ J. Elks, ed. (2014). The Dictionary of Drugs: Chemical Data, Structures and Bibliographies. Springer.
- ^ Pallos, L.; Budai, Z.; Zólyomi, G. (1972). "Basic ethers of 1-substituted cycloalkanols. 1". Arzneimittel-Forschung. 22 (9): 1502–1505. PMID 4678613.
- ^ Pallos, L.; Budai, Z.; Zólyomi, G. (1972). "Basic ethers of 1-substituted cycloalkanols. 2". Arzneimittel-Forschung. 22 (9): 1505–1509. PMID 4678614.
- ^ Anon., ES 376120 (1972-04-01 to Gallardo Antonio SA).
- ^ Spaeter Genannt Werden Wird, DE 2455051A1 (1975 to Andreu S A Lab).
