Clavulanic acid
 | |
 | |
| Clinical data | |
|---|---|
| Pronunciation | /ˌklævjʊˈlænɪk/ |
| Other names | RX-10100; Serdaxin; Zoraxel |
| AHFS/Drugs.com | International Drug Names |
| Pregnancy category |
|
| Routes of administration | Oral, IV |
| ATC code |
|
| Legal status | |
| Legal status |
|
| Pharmacokinetic data | |
| Bioavailability | Oral: 45–64%[1][2] |
| Protein binding | ~25%[2] |
| Metabolism | Unknown[1] |
| Metabolites | Two minor metabolites[2] |
| Onset of action | ≤0.67–2 hours (Tmax)[2] |
| Elimination half-life | 0.8–1.2 hours[1][2] |
| Excretion | Urine: 35–65% (unchanged; within 6 hours)[1][2] |
| Identifiers | |
| |
| CAS Number | |
| PubChem CID | |
| DrugBank | |
| ChemSpider | |
| UNII | |
| KEGG | |
| ChEBI | |
| ChEMBL | |
| CompTox Dashboard (EPA) | |
| ECHA InfoCard | 100.055.500 |
| Chemical and physical data | |
| Formula | C8H9NO5 |
| Molar mass | 199.162 g·mol−1 |
| 3D model (JSmol) | |
| |
| |
| (verify) | |
Clavulanic acid is a β-lactam drug that functions as a mechanism-based β-lactamase inhibitor. While not effective by itself as an antibiotic, when combined with penicillin-group antibiotics, it can overcome antibiotic resistance in bacteria that secrete β-lactamase, which otherwise inactivates most penicillins.
In its most common preparations, potassium clavulanate (clavulanic acid as a salt of potassium) is combined with:
- amoxicillin (co-amoxiclav, trade names Augmentin, Clavulin, Tyclav, Clavaseptin (veterinary), Clavamox (veterinary), Synulox (veterinary), and others)
- ticarcillin (co-ticarclav, trade name Timentin)
Clavulanic acid was patented in 1974.[3] In addition to its β-lactamase inhibition, clavulanic acid shows off-target activity in the nervous system by upregulating the glutamate transporter 1 (GLT-1) and has been studied in the potential treatment of a variety of central nervous system disorders.[1][4]
Medical uses
[edit]Amoxicillin–clavulanic acid is a first-line treatment for many types of infections, including sinus infections, and urinary tract infections, including pyelonephritis. This is, in part, because of its efficacy against gram-negative bacteria which tend to be more difficult to control than gram-positive bacteria with chemotherapeutic antibiotics.[clarification needed]
Adverse effects
[edit]The use of clavulanic acid with penicillins has been associated with an increased incidence of cholestatic jaundice and acute hepatitis during therapy or shortly after. The associated jaundice is usually self-limiting and very rarely fatal.[5][6]
The UK Committee on Safety of Medicines (CSM) recommends that treatments such as amoxicillin/clavulanic acid preparations be reserved for bacterial infections likely to be caused by amoxicillin-resistant β-lactamase-producing strains, and that treatment should not normally exceed 14 days.
Allergic reactions have been reported.[7]
Sources
[edit]The name is derived from strains of Streptomyces clavuligerus, which produces clavulanic acid.[8][9]
Biosynthesis
[edit]
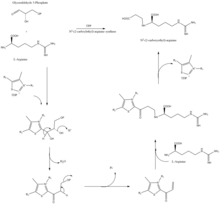
The β-lactam like structure of clavulanic acid looks structurally similar to penicillin, but the biosynthesis of this molecule involves a different biochemical pathway. Clavulanic acid is produced by the bacterium Streptomyces clavuligerus, using glyceraldehyde-3-phosphate and L-arginine as starting materials.[10][11] Although each of the intermediates of the pathway is known, the exact mechanism for all of the enzymatic reactions is not fully understood. The process mainly involves 3 enzymes: clavaminate synthase, β-lactam synthetase, and N2-(2-carboxyethyl)-L-arginine (CEA) synthase.[10] Clavaminate synthase is a non-heme oxygenase dependent on iron and α-keto-glutarate and is encoded by orf5 of the clavulanic acid gene cluster. The specific mechanism of how this enzyme works is not fully understood, but this enzyme regulates 3 steps in the overall synthesis of clavulanic acid. All 3 steps occur in the same region of the catalytic, iron-containing reaction center, yet do not occur in sequence and affect different areas of the clavulanic acid structure.[12]
β-lactam synthetase is a 54.5 kDa protein that is encoded by orf3 of the clavulanic acid gene cluster, and shows similarity to asparagine synthase – Class B enzymes. The exact mechanism on how this enzyme works to synthesize the β-lactam is not proven, but is believed to occur in coordination with a CEA synthase and ATP.[13]

CEA synthase is a 60.9 kDA protein and is the first gene found in the clavulanic acid biosynthesis gene cluster, encoded by orf2 of the clavulanic acid gene cluster. The specific mechanism of how this enzyme works is still under investigation; however, it is known that this enzyme has the ability to couple together glyceraldehyde-3-phosphate with L-arginine in the presence of thiamine diphosphate (TDP or thiamine pyrophosphate), which is the first step of the clavulanic acid biosynthesis.[14]
History
[edit]Clavulanic acid was discovered around 1974-75 by British scientists working at the drug company Beecham from the bacteria Streptomyces clavuligerus.[15] After several attempts, Beecham finally filed for US patent protection for the drug in 1981, and U.S. Patents 4,525,352, 4,529,720, and 4,560,552 were granted in 1985.
Clavulanic acid has negligible intrinsic antimicrobial activity, despite sharing the β-lactam ring that is characteristic of β-lactam antibiotics. However, the similarity in chemical structure allows the molecule to interact with the enzyme β-lactamase secreted by certain bacteria to confer resistance to β-lactam antibiotics.
Clavulanic acid is a suicide inhibitor, covalently bonding to a serine residue in the active site of the β-lactamase. This restructures the clavulanic acid molecule, creating a much more reactive species that attacks another amino acid in the active site, permanently inactivating it, and thus inactivating the enzyme.
This inhibition restores the antimicrobial activity of β-lactam antibiotics against lactamase-secreting resistant bacteria. Despite this, some bacterial strains that are resistant even to such combinations have emerged.
Research
[edit]Neuromodulation
[edit]In 2005, it was discovered via screening of 1,040 Food and Drug Administration (FDA)-approved drugs and neutraceuticals that many β-lactams, such as ceftriaxone, upregulate astrocytic glutamate transporter 1 (GLT-1) expression.[1][16][17] Subsequently, it was discovered that clavulanic acid, likewise a β-lactam, shares this action.[1][18] The associated effects include enhanced GLT-1 expression in the nucleus accumbens, medial prefrontal cortex, and spinal cord, modulation of glutamatergic, dopaminergic, and serotonergic neurotransmission, and anti-inflammatory effects via modulation of cytokines tumor necrosis factor α (TNF-α) and interleukin-10 (IL-10).[1][19][4] Ceftriaxone lacks oral bioavailability, has poor brain permeability, and has concomitant antibiotic activity.[1] These limitations have resulted in more interest in clavulanic acid, which does not share these drawbacks and is more potent than ceftriaxone in vivo.[1] The mechanism of action underlying the upregulation of GLT-1 expression by β-lactams is unknown.[1][17] However, interactions with the SNARE proteins Munc18-1 and Rab4 may be involved in some of clavulanic acid's effects, such as increased dopamine release.[20][21]
In relation to its central nervous system actions, clavulanic acid has been studied preclinically in models of anxiety, sexual behavior, addiction, neuropathic pain, inflammatory pain, epilepsy, Parkinson's disease, dementia, and stroke.[1][19][22][20] In animals, including in rodents and/or monkeys, clavulanic acid has shown anxiolytic-like, antidepressant-like, pro-sexual, memory-enhancing, analgesic, antiaddictive, pro-dopaminergic, pro-oxytocinergic, and neuroprotective effects.[1][20][18][23] The drug has been studied clinically in humans in the treatment of erectile dysfunction,[19] depression,[24][25][26] substance dependence,[27] and pain,[20] with positive or mixed preliminary results for these conditions reported.[4][19][24][26]
Clavulanic acid was under formal development by Revaax Pharmaceuticals (now Ocuphire Pharma) for the treatment of erectile dysfunction, anxiety disorders, major depressive disorder, neurodegenerative disorders, and Parkinson's disease.[4][19][24] However, development for these indications was discontinued by 2014.[4] The developmental code name of clavulanic acid was RX-10100 and its tentative brand names were Serdaxin and Zoraxel.[4] Although its development was discontinued, interest in clavulanic acid for potential nervous system-related uses has continued as of 2024.[1][27]
References
[edit]- ^ a b c d e f g h i j k l m n Balcazar-Ochoa LG, Ventura-Martínez R, Ángeles-López GE, Gómez-Acevedo C, Carrasco OF, Sampieri-Cabrera R, Chavarría A, González-Hernández A (January 2024). "Clavulanic Acid and its Potential Therapeutic Effects on the Central Nervous System". Arch Med Res. 55 (1): 102916. doi:10.1016/j.arcmed.2023.102916. PMID 38039802.
- ^ a b c d e f "Clavulanic acid: Uses, Interactions, Mechanism of Action". DrugBank Online. 8 July 2014. Retrieved 5 November 2024.
- ^ Fischer J, Ganellin CR (2006). Analogue-based Drug Discovery. John Wiley & Sons. p. 490. ISBN 9783527607495.
- ^ a b c d e f "Clavulanic acid". AdisInsight. 29 December 2021. Retrieved 27 September 2024.
- ^ Joint Formulary Committee. British National Formulary, 47th edition. London: British Medical Association and Royal Pharmaceutical Society of Great Britain; 2004.
- ^ "Drug Record - Amoxicillin-Clavulanate". LiverTox - Clinical and Research Information on Drug-Induced Liver Injury. 2012. PMID 31643176. Archived from the original on November 23, 2016. Retrieved April 24, 2013.
- ^ Tortajada Girbés M, Ferrer Franco A, Gracia Antequera M, Clement Paredes A, García Muñoz E, Tallón Guerola M (2008). "Hypersensitivity to clavulanic acid in children". Allergologia et Immunopathologia. 36 (5): 308–310. doi:10.1016/S0301-0546(08)75228-5. PMID 19080805. Archived from the original on 2012-04-07. Retrieved 2011-11-11.
- ^ Arulanantham H, Kershaw NJ, Hewitson KS, Hughes CE, Thirkettle JE, Schofield CJ (January 2006). "ORF17 from the clavulanic acid biosynthesis gene cluster catalyzes the ATP-dependent formation of N-glycyl-clavaminic acid". The Journal of Biological Chemistry. 281 (1): 279–287. doi:10.1074/jbc.M507711200. PMID 16251194.
- ^ Tahlan K, Park HU, Wong A, Beatty PH, Jensen SE (March 2004). "Two sets of paralogous genes encode the enzymes involved in the early stages of clavulanic acid and clavam metabolite biosynthesis in Streptomyces clavuligerus". Antimicrobial Agents and Chemotherapy. 48 (3): 930–939. doi:10.1128/AAC.48.3.930-939.2004. PMC 353097. PMID 14982786.
- ^ a b c d e Townsend CA (October 2002). "New reactions in clavulanic acid biosynthesis". Current Opinion in Chemical Biology. 6 (5): 583–589. doi:10.1016/S1367-5931(02)00392-7. PMID 12413541.
- ^ Reading C, Cole M (May 1977). "Clavulanic acid: a beta-lactamase-inhiting beta-lactam from Streptomyces clavuligerus". Antimicrobial Agents and Chemotherapy. 11 (5): 852–857. doi:10.1128/AAC.11.5.852. PMC 352086. PMID 879738.
- ^ Busby RW, Townsend CA (July 1996). "A single monomeric iron center in clavaminate synthase catalyzes three nonsuccessive oxidative transformations". Bioorganic & Medicinal Chemistry. 4 (7): 1059–1064. doi:10.1016/0968-0896(96)00088-0. PMID 8831977.
- ^ Bachmann BO, Townsend CA (September 2000). "Kinetic mechanism of the beta-lactam synthetase of Streptomyces clavuligerus". Biochemistry. 39 (37): 11187–11193. doi:10.1021/bi000709i. PMID 10985764.
- ^ Khaleeli N, Li R, Townsend CA (1999). "Origin of the β-Lactam Carbons in Clavulanic Acid from an Unusual Thiamine Pyrophosphate-Mediated Reaction". Journal of the American Chemical Society. 121 (39): 9223–9224. doi:10.1021/ja9923134.
- ^ Sutherland R (June 1991). "Beta-lactamase inhibitors and reversal of antibiotic resistance". Trends in Pharmacological Sciences. 12 (6): 227–232. doi:10.1016/0165-6147(91)90557-9. PMID 2048218.
- ^ Abulseoud OA, Alasmari F, Hussein AM, Sari Y (2022). "Ceftriaxone as a Novel Therapeutic Agent for Hyperglutamatergic States: Bridging the Gap Between Preclinical Results and Clinical Translation". Front Neurosci. 16: 841036. doi:10.3389/fnins.2022.841036. PMC 9294323. PMID 35864981.
- ^ a b Rothstein JD, Patel S, Regan MR, Haenggeli C, Huang YH, Bergles DE, Jin L, Dykes Hoberg M, Vidensky S, Chung DS, Toan SV, Bruijn LI, Su ZZ, Gupta P, Fisher PB (January 2005). "Beta-lactam antibiotics offer neuroprotection by increasing glutamate transporter expression". Nature. 433 (7021): 73–77. Bibcode:2005Natur.433...73R. doi:10.1038/nature03180. PMID 15635412.
- ^ a b Kim DJ, King JA, Zuccarelli L, Ferris CF, Koppel GA, Snowdon CT, Ahn CH (August 2009). "Clavulanic acid: a competitive inhibitor of beta-lactamases with novel anxiolytic-like activity and minimal side effects". Pharmacol Biochem Behav. 93 (2): 112–120. doi:10.1016/j.pbb.2009.04.013. PMID 19394358.
- ^ a b c d e Milenkovic U, Campbell J, Roussel E, Albersen M (December 2018). "An update on emerging drugs for the treatment of erectile dysfunction". Expert Opin Emerg Drugs. 23 (4): 319–330. doi:10.1080/14728214.2018.1552938. PMID 30507329.
- ^ a b c d Ochoa-Aguilar A, Ventura-Martinez R, Sotomayor-Sobrino MA, Gómez C, Morales-Espinoza MR (2016). "Review of Antibiotic and Non-Antibiotic Properties of Beta-lactam Molecules". Anti-Inflamm Anti-Allergy Agents Med Chem. 15 (1): 3–14. doi:10.2174/1871523015666160517114027. PMID 27185396.
- ^ Kost GC, Selvaraj S, Lee YB, Kim DJ, Ahn CH, Singh BB (October 2011). "Clavulanic acid increases dopamine release in neuronal cells through a mechanism involving enhanced vesicle trafficking". Neurosci Lett. 504 (2): 170–175. doi:10.1016/j.neulet.2011.09.032. PMC 3195833. PMID 21964384.
- ^ Esmaili-Shahzade-Ali-Akbari P, Ghaderi A, Hosseini SM, Nejat F, Saeedi-Mofrad M, Karimi-Houyeh M, Ghattan A, Etemadi A, Rasoulian E, Khezri A (November 2023). "β_lactam antibiotics against drug addiction: A novel therapeutic option". Drug Dev Res. 84 (7): 1411–1426. doi:10.1002/ddr.22110. PMID 37602907.
- ^ Arab AO, Alasmari F, Albaker AB, Alhazmi HA, Alameen AA, Alagail NM, Alwaeli SA, Rizwan Ahamad S, AlAsmari AF, AlSharari SD (October 2023). "Clavulanic Acid Improves Memory Dysfunction and Anxiety Behaviors through Upregulating Glutamatergic Transporters in the Nucleus Accumbens of Mice Repeatedly Exposed to Khat Extract". Int J Mol Sci. 24 (21): 15657. doi:10.3390/ijms242115657. PMC 10648086. PMID 37958641.
- ^ a b c Connolly KR, Thase ME (March 2012). "Emerging drugs for major depressive disorder". Expert Opin Emerg Drugs. 17 (1): 105–126. doi:10.1517/14728214.2012.660146. PMID 22339643.
- ^ Belzung C (April 2014). "Innovative drugs to treat depression: did animal models fail to be predictive or did clinical trials fail to detect effects?". Neuropsychopharmacology. 39 (5): 1041–1051. doi:10.1038/npp.2013.342. PMC 3957126. PMID 24345817.
- ^ a b Riesenberg R, Rosenthal J, Moldauer L, Peterson C (June 2012). "Results of a proof-of-concept, dose-finding, double-blind, placebo-controlled study of RX-10100 (Serdaxin®) in subjects with major depressive disorder". Psychopharmacology (Berl). 221 (4): 601–610. doi:10.1007/s00213-011-2604-x. PMID 22203317.
- ^ a b Callans LS, Philogene-Khalid H, Jagannathan K, Cunningham R, Yu D, Lu X, Walters MI, Morrison MF (April 2024). "Clavulanic Acid Decreases Cocaine Cue Reactivity in Addiction-Related Brain Areas, a Randomized fMRI Pilot Study". Psychopharmacol Bull. 54 (2): 8–14. PMC 11003254. PMID 38601830.
