Zingerone

| |
| Names | |
|---|---|
| Preferred IUPAC name
4-(4-Hydroxy-3-methoxyphenyl)butan-2-one | |
| Identifiers | |
3D model (JSmol)
|
|
| ChEBI | |
| ChEMBL | |
| ChemSpider | |
| ECHA InfoCard | 100.004.136 |
PubChem CID
|
|
| UNII | |
CompTox Dashboard (EPA)
|
|
| |
| |
| Properties | |
| C11H14O3 | |
| Molar mass | 194.22 g/mol |
| Melting point | 40 to 41 °C (104 to 106 °F; 313 to 314 K) |
| Boiling point | 187 to 188 °C (369 to 370 °F; 460 to 461 K) at 14 mmHg |
| Insoluble | |
| Solubility | Miscible in ether |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
| |
Zingerone, also called vanillylacetone, is a major flavor component of ginger, providing the sweet flavor of cooked ginger.[1] Zingerone is a crystalline solid that is sparingly soluble in water and soluble in ether.
Zingerone is similar in chemical structure to other flavor chemicals such as vanillin and eugenol. It is used as a flavor additive in spice oils and in perfumery to introduce spicy aromas.
Fresh ginger does not contain zingerone, but it is produced by cooking or drying of the ginger root, which causes a reverse aldol reaction on gingerol.
Production
[edit]History
[edit]Zingerone was first isolated from the ginger root in 1917 by Hiroshi Nomura, a chemistry professor at Tokyo Imperial University.[2] Nomura named the compound and identified the empirical formula of zingerone in his studies at the laboratory of the Agricultural College. He initially identified it as the chemical component contributing pungency to ginger, something further work has disproven. [3]
Current methods
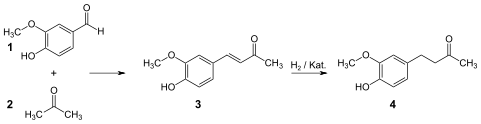
[edit]Nomura identified and later patented a method for the synthesis of zingerone, in which vanillin and acetone are reacted under basic conditions to form dehydrozingerone. This compound is obtained in about 95% quantity.[4] This reaction is followed by catalytic hydrogenation of the intermediate compound in order to form zingerone, obtained in approximately 100% quantity.[5]
Biological effects
[edit]This section needs more reliable medical references for verification or relies too heavily on primary sources. (November 2019) |  |
Ginger compounds have been shown to be active against enterotoxigenic Escherichia coli heat-labile enterotoxin-induced diarrhea. This type of diarrhea is the leading cause of infant death in developing countries. Zingerone is likely the active constituent responsible for the antidiarrheal efficacy of ginger.[6]
Zingerone is recognized as being a particularly efficient free radical scavenger. It is able to scavenge and degrade free radicals and reactive oxygen species in the body, and inhibits enzymes involved in the generation of these reactive oxygen species.[7]
It is used by some flowers to attract pollinating fruit flies by mimicking the sex pheromone of the fly.[8][9]
References
[edit]- ^ Monge, P; Scheline, R; Solheim, E (1976). "The metabolism of zingerone, a pungent principle of ginger". Xenobiotica. 6 (7): 411–23. doi:10.3109/00498257609151654. PMID 997589.
- ^ Nomura, Hiroshi (1917). "The pungent principles of ginger. Part I. A new ketone, zingerone (4-hydroxy-3-methoxyphenylethyl methyl ketone) occurring in ginger". Journal of the Chemical Society, Transactions. 111: 769–776. doi:10.1039/ct9171100769.
- ^ Steffen Arctander, Perfume and Flavor Materials of Natural Origin, pg. 280
- ^ Wu, Anxin; Wang, Zihua; Yin, Guodong; Qin, Jing; Gao, Meng; Cao, Liping (2008). "An Efficient Method for the Selective Iodination of α,β-Unsaturated Ketones". Synthesis. 2008 (22): 3675–3681. doi:10.1055/s-0028-1083200.
- ^ Nomura, Hiroshi. "Method of preparing 'zingerone' (methyl-3-methoxy-4-hydroxyphenyl-ethyl ketone." U.S. Patent 1,263,796. Issued April 23, 1918.
- ^ Chen, Jaw-Chyun; Li-Jiau Huang; Shih-Lu Wu; Sheng-Chu Kuo; Tin-Yun Ho; Chien-Yun Hsiang (2007). "Ginger and Its Bioactive Component Inhibit Enterotoxigenic Escherichia coli Heat-Labile Enterotoxin-Induced Diarrhea in Mice". Journal of Agricultural and Food Chemistry. 55 (21): 8390–7. doi:10.1021/jf071460f. PMID 17880155.
- ^ Rajan, Iyappan; Narayanan, Nithya; Rabindran, Remitha; Jayasree, P. R.; Kumar, P. R. Manish (2013-12-01). "Zingerone Protects Against Stannous Chloride-Induced and Hydrogen Peroxide-Induced Oxidative DNA Damage In Vitro". Biological Trace Element Research. 155 (3): 455–459. Bibcode:2013BTER..155..455R. doi:10.1007/s12011-013-9801-x. ISSN 0163-4984. PMID 24006104. S2CID 17920702.
- ^ Tan, Keng-hong; Nishida, Ritsuo (2000). "Mutual Reproductive Benefits Between a Wild Orchid, Bulbophyllum patens, and Bactrocera Fruit Flies via a Floral Synomone". Journal of Chemical Ecology. 26 (2): 533–546. Bibcode:2000JCEco..26..533T. doi:10.1023/A:1005477926244. S2CID 24971928.
- ^ Tan, Keng-Hong; Nishida, Ritsuo (2007). "Zingerone in the floral synomone of Bulbophyllum baileyi (Orchidaceae) attracts Bactrocera fruit flies during pollination". Biochemical Systematics and Ecology. 35 (6): 334–341. Bibcode:2007BioSE..35..334T. doi:10.1016/j.bse.2007.01.013.