Sitagliptin
 | |
 | |
| Clinical data | |
|---|---|
| Pronunciation | /sɪtəˈɡlɪptɪn/ |
| Trade names | Januvia, Zituvio, others |
| AHFS/Drugs.com | Monograph |
| MedlinePlus | a606023 |
| License data |
|
| Pregnancy category |
|
| Routes of administration | By mouth |
| ATC code | |
| Legal status | |
| Legal status | |
| Pharmacokinetic data | |
| Bioavailability | 87% |
| Protein binding | 38% |
| Metabolism | Liver (CYP3A4- and CYP2C8-mediated) |
| Elimination half-life | 8 to 14 h[7] |
| Excretion | Kidney (80%)[7] |
| Identifiers | |
| |
| CAS Number | |
| PubChem CID | |
| IUPHAR/BPS | |
| DrugBank | |
| ChemSpider | |
| UNII | |
| KEGG | |
| ChEBI | |
| ChEMBL | |
| CompTox Dashboard (EPA) | |
| ECHA InfoCard | 100.217.948 |
| Chemical and physical data | |
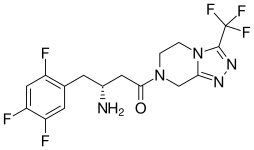
| Formula | C16H15F6N5O |
| Molar mass | 407.320 g·mol−1 |
| 3D model (JSmol) | |
| |
| |
| (verify) | |
Sitagliptin, sold under the brand name Januvia among others, is an anti-diabetic medication used to treat type 2 diabetes.[8] In the United Kingdom it is listed as less preferred than metformin or a sulfonylurea.[9] It is taken by mouth.[8] It is also available in the fixed-dose combination medication sitagliptin/metformin (Janumet, Janumet XR).[8]
Common side effects include headaches, swelling of the legs, and upper respiratory tract infections.[8] Serious side effects may include angioedema, low blood sugar, kidney problems, pancreatitis, and joint pain.[8] Whether use in pregnancy or breastfeeding is safe is unclear.[10] It is in the dipeptidyl peptidase-4 (DPP-4) inhibitor class and works by increasing the production of insulin and decreasing the production of glucagon by the pancreas.[8]
Sitagliptin was developed by Merck & Co. and approved for medical use in the United States in 2006.[8] In 2022, it was the 86th most commonly prescribed medication in the United States, with more than 7 million prescriptions.[11][12] It is available as a generic medication.[13][14][15]
Medical uses
[edit]Sitagliptin is used to treat type 2 diabetes.[8] It is generally less preferred than metformin or sulfonylureas.[9] It is taken by mouth.[8] It is also available as the fixed-dose combinations of sitagliptin/metformin (Janumet, Janumet XR)[8] and sitagliptin/simvastatin (Juvisync).[16]
Sitagliptin should not be used to treat type 1 diabetes. In December 2020, the US Food and Drug Administration (FDA) approved labeling changes stating that Januvia (sitagliptin), Janumet (sitagliptin and metformin hydrochloride), and Janumet XR (sitagliptin and metformin hydrochloride extended-release) are not proven to improve glycemic (blood sugar) control in children aged 10 to 17 with type 2 diabetes.[17] The drugs are approved to improve blood sugar control in adults aged 18 and older with type 2 diabetes.[17]
Adverse effects
[edit]Adverse effects from sitagliptin are similar to placebo, except for rare nausea, common cold-like symptoms, and photosensitivity.[18] It does not increase the risk of diarrhea.[19] No significant difference exists in the occurrence of hypoglycemia between placebo and sitagliptin.[18][20][21] In those taking sulphonylureas, the risk of low blood sugar is increased.[22]
The existence of rare case reports of kidney failure and hypersensitivity reactions is noted in the United States prescribing information, but a causative role for sitagliptin has not been established.[2]
Several postmarketing reports of pancreatitis (some fatal) have been made in people treated with sitagliptin and other DPP-4 inhibitors,[23][24] and the US FDA package insert carries a warning to this effect,[2] although the causal link between sitagliptin and pancreatitis has not yet been fully substantiated.[25] One study with lab rats published in 2009 concluded that some of the possible risks of pancreatitis or pancreatic cancer may be reduced when it is used with metformin. However, while DPP-4 inhibitors showed an increase in such risk factors, as of 2009, no increase in pancreatic cancer has been reported in individuals taking DPP-4 inhibitors.[26]
In 2015, the US Food and Drug Administration (FDA) added a new warning and precaution about the risk of "severe and disabling" joint pain to the labels of all DPP-4 inhibitor medicines.[27]
Mechanism of action
[edit]Sitagliptin works to competitively inhibit the enzyme dipeptidyl peptidase 4 (DPP-4). This enzyme breaks down the incretins GLP-1 and GIP, gastrointestinal hormones released in response to a meal.[28] By preventing breakdown of GLP-1 and GIP, they are able to increase the secretion of insulin and suppress the release of glucagon by the alpha cells of the pancreas.[medical citation needed] This drives blood glucose levels towards normal.[medical citation needed] As the blood glucose level approaches normal, the amounts of insulin released and glucagon suppressed diminishes, thus tending to prevent an "overshoot" and subsequent low blood sugar (hypoglycemia), which is seen with some other oral hypoglycemic agents.[medical citation needed]
Sitagliptin has been shown to lower HbA1c level by about 0.7% points versus placebo. It is slightly less effective than metformin when used as a monotherapy. It does not cause weight gain and has less hypoglycemia compared to sulfonylureas. Sitagliptin is recommended as a second-line drug (in combination with other drugs) after the combination of diet/exercise and metformin fails.[29]
History
[edit]Sitagliptin was approved by the US Food and Drug Administration (FDA) in October 2006,[30] and is sold under the brand name Januvia.[31] In April 2007, the FDA approved an oral combination of sitagliptin/metformin sold under the brand name Janumet.[32] In October 2011, the FDA approved an oral combination of sitagliptin/simvastatin sold under the brand name Juvisync.[33][16] The extended release version of sitagliptin/metformin was approved in February 2012.[34]
External links
[edit] Media related to Sitagliptin at Wikimedia Commons
Media related to Sitagliptin at Wikimedia Commons
References
[edit]- ^ "Product monograph brand safety updates". Health Canada. February 2024. Retrieved March 24, 2024.
- ^ a b c "Januvia- sitagliptin tablet, film coated". DailyMed. Archived from the original on October 27, 2021. Retrieved October 15, 2021.
- ^ "Zituvio- sitagliptin tablet". DailyMed. November 1, 2023. Retrieved December 25, 2023.
- ^ "Zituvio- sitagliptin tablet". DailyMed. November 1, 2023. Retrieved December 25, 2023.
- ^ "Januvia EPAR". European Medicines Agency. September 17, 2018. Archived from the original on October 23, 2021. Retrieved October 15, 2021.
- ^ "Xelevia EPAR". European Medicines Agency (EMA). March 21, 2007. Retrieved October 19, 2024.
- ^ a b Herman GA, Stevens C, van Dyck K, Bergman A, Yi B, De Smet M, et al. (December 2005). "Pharmacokinetics and pharmacodynamics of sitagliptin, an inhibitor of dipeptidyl peptidase IV, in healthy subjects: results from two randomized, double-blind, placebo-controlled studies with single oral doses". Clinical Pharmacology and Therapeutics. 78 (6): 675–688. doi:10.1016/j.clpt.2005.09.002. PMID 16338283. S2CID 20935646.
{{cite journal}}: CS1 maint: overridden setting (link) - ^ a b c d e f g h i j "Sitagliptin Monograph for Professionals". Drugs.com. American Society of Health-System Pharmacists. Archived from the original on March 4, 2016. Retrieved March 3, 2019.
- ^ a b British national formulary : BNF 76 (76 ed.). Pharmaceutical Press. 2018. p. 681. ISBN 9780857113382.
- ^ "Sitagliptin Pregnancy and Breastfeeding Warnings". Drugs.com. Archived from the original on March 6, 2019. Retrieved March 3, 2019.
- ^ "The Top 300 of 2022". ClinCalc. Archived from the original on August 30, 2024. Retrieved August 30, 2024.
- ^ "Sitagliptin Drug Usage Statistics, United States, 2013 - 2022". ClinCalc. Retrieved August 30, 2024.
- ^ "Generic Januvia Availability". Drugs.com. Retrieved December 1, 2023.
- ^ "JAMP Pharma Group receives Health Canada approval for PrJAMP Sitagliptin, a new generic alternative for the treatment of type 2 diabetes" (Press release). JAMP Pharma. January 6, 2023. Retrieved June 19, 2023 – via Newswire.
- ^ "Sitagliptin SUN EPAR". European Medicines Agency (EMA). December 9, 2021. Retrieved September 27, 2024.
- ^ a b "FDA Approves Combination Therapy Juvisync" (Press release). U.S. Food and Drug Administration (FDA). October 7, 2011. Archived from the original on August 24, 2014. Retrieved November 17, 2013.
- ^ a b "Diabetes drug not proven to improve blood sugar in pediatric patients". U.S. Food and Drug Administration (FDA). December 4, 2020. Archived from the original on December 4, 2020. Retrieved December 5, 2020.
 This article incorporates text from this source, which is in the public domain.
This article incorporates text from this source, which is in the public domain.
- ^ a b "Januvia Side Effects & Drug Interactions". RxList.com. 2007. Archived from the original on November 20, 2007. Retrieved November 28, 2007.
- ^ Zhao Q, Hong D, Zheng D, Xiao Y, Wu B (2014). "Risk of diarrhea in patients with type 2 diabetes mellitus treated with sitagliptin: a meta-analysis of 30 randomized clinical trials". Drug Design, Development and Therapy. 8: 2283–2294. doi:10.2147/DDDT.S70945. PMC 4234286. PMID 25419118.
- ^ Stricklin SM, Stoecker WV, Rader RK, Hood AF, Litt JZ, Schuman TP (February 2012). "Persistent edematous-plaque photosensitivity observed with sitagliptin phosphate (Januvia®)". Dermatology Online Journal. 18 (2): 9. doi:10.5070/D30D70K7B2. PMID 22398230. Archived from the original on April 8, 2019. Retrieved June 6, 2019.
- ^ "Januvia side effect: Photosensitivity reaction - eHealthMe". www.ehealthme.com. Archived from the original on June 7, 2019. Retrieved June 6, 2019.
- ^ Salvo F, Moore N, Arnaud M, Robinson P, Raschi E, De Ponti F, et al. (May 2016). "Addition of dipeptidyl peptidase-4 inhibitors to sulphonylureas and risk of hypoglycaemia: systematic review and meta-analysis". BMJ. 353: i2231. doi:10.1136/bmj.i2231. PMC 4854021. PMID 27142267.
{{cite journal}}: CS1 maint: overridden setting (link) - ^ Olansky L (January 2010). "Do incretin-based therapies cause acute pancreatitis?". Journal of Diabetes Science and Technology. 4 (1): 228–229. doi:10.1177/193229681000400129. PMC 2825646. PMID 20167189.
- ^ "FDA Drug Safety Communication: FDA investigating reports of possible increased risk of pancreatitis and pre-cancerous findings of the pancreas from incretin mimetic drugs for type 2 diabetes". U.S. Food and Drug Administration (FDA). June 21, 2019. Archived from the original on May 10, 2022. Retrieved May 10, 2022.
- ^ National Prescribing Service (August 2010). "Sitagliptin for Type 2 Diabetes". Archived from the original on July 18, 2010. Retrieved August 27, 2010.
- ^ Matveyenko AV, Dry S, Cox HI, Moshtaghian A, Gurlo T, Galasso R, et al. (July 2009). "Beneficial endocrine but adverse exocrine effects of sitagliptin in the human islet amyloid polypeptide transgenic rat model of type 2 diabetes: interactions with metformin". Diabetes. 58 (7): 1604–1615. doi:10.2337/db09-0058. PMC 2699878. PMID 19403868.
{{cite journal}}: CS1 maint: overridden setting (link) - ^ "DPP-4 Inhibitors for Type 2 Diabetes: Drug Safety Communication—May Cause Severe Joint Pain". U.S. Food and Drug Administration (FDA). August 28, 2015. Archived from the original on December 13, 2019. Retrieved September 1, 2015.
- ^ Herman GA, Bergman A, Liu F, Stevens C, Wang AQ, Zeng W, et al. (August 2006). "Pharmacokinetics and pharmacodynamic effects of the oral DPP-4 inhibitor sitagliptin in middle-aged obese subjects". Journal of Clinical Pharmacology. 46 (8): 876–886. doi:10.1177/0091270006289850. PMID 16855072. S2CID 45849328.
{{cite journal}}: CS1 maint: overridden setting (link) - ^ Gadsby R (2009). "Efficacy and Safety of Sitagliptin in the Treatment of Type 2 Diabetes". Clinical Medicine: Therapeutics. 1 (1): 53–62. doi:10.4137/CMT.S2313.
- ^ "FDA Approves New Treatment for Diabetes" (Press release). U.S. Food and Drug Administration (FDA). October 17, 2006. Archived from the original on February 28, 2009. Retrieved October 17, 2006.
- ^ "Drug Approval Package: Januvia (Sitagliptin Phosphate) NDA #021995". U.S. Food and Drug Administration (FDA). Retrieved September 27, 2024.
- ^ "Drug Approval Package: Janumet (Sitagliptin/Metformin Hydrochloride) NDA #022044". U.S. Food and Drug Administration (FDA). July 8, 2008. Retrieved September 27, 2024.
- ^ "Drug Approval Package: Juvisync (sitagliptin and simvastatin fixed-dose combination) Tablets NDA #202343". U.S. Food and Drug Administration (FDA). July 13, 2012. Retrieved September 27, 2024.
- ^ "Drug Approval Package: Janumet XR (sitagliptin/metformin hydrochloride) NDA #202270". U.S. Food and Drug Administration (FDA). September 3, 2013. Retrieved September 27, 2024.
