Horner–Wadsworth–Emmons reaction
| Horner–Wadsworth–Emmons reaction | |
|---|---|
| Named after | Leopold Horner William S. Wadsworth William D. Emmons |
| Reaction type | Coupling reaction |
| Identifiers | |
| Organic Chemistry Portal | wittig-horner-reaction |
| RSC ontology ID | RXNO:0000056 |
The Horner–Wadsworth–Emmons (HWE) reaction is a chemical reaction used in organic chemistry of stabilized phosphonate carbanions with aldehydes (or ketones) to produce predominantly E-alkenes.[1]

In 1958, Leopold Horner published a modified Wittig reaction using phosphonate-stabilized carbanions.[2][3] William S. Wadsworth and William D. Emmons further defined the reaction.[4][5]
In contrast to phosphonium ylides used in the Wittig reaction, phosphonate-stabilized carbanions are more nucleophilic but less basic. Likewise, phosphonate-stabilized carbanions can be alkylated. Unlike phosphonium ylides, the dialkylphosphate salt byproduct is easily removed by aqueous extraction.
Several reviews have been published.[6][7][8][9][10][11]
Reaction mechanism
[edit]
The Horner–Wadsworth–Emmons reaction begins with the deprotonation of the phosphonate to give the phosphonate carbanion 1. Nucleophilic addition of the carbanion onto the aldehyde 2 (or ketone) producing 3a or 3b is the rate-limiting step.[12] If R2 = H, then intermediates 3a and 4a and intermediates 3b and 4b can interconvert with each other.[13] The final elimination of oxaphosphetanes 4a and 4b yield (E)-alkene 5 and (Z)-alkene 6, with the by-product being a dialkyl-phosphate.

The ratio of alkene isomers 5 and 6 is not dependent upon the stereochemical outcome of the initial carbanion addition and upon the ability of the intermediates to equilibrate.
The electron-withdrawing group (EWG) alpha to the phosphonate is necessary for the final elimination to occur. In the absence of an electron-withdrawing group, the final product is the β-hydroxyphosphonate 3a and 3b.[14] However, these β-hydroxyphosphonates can be transformed to alkenes by reaction with diisopropylcarbodiimide.[15]
Stereoselectivity
[edit]The Horner–Wadsworth–Emmons reaction favours the formation of (E)-alkenes. In general, the more equilibration amongst intermediates, the higher the selectivity for (E)-alkene formation.
Disubstituted alkenes
[edit]Thompson and Heathcock have performed a systematic study of the reaction of methyl 2-(dimethoxyphosphoryl)acetate with various aldehydes.[16] While each effect was small, they had a cumulative effect making it possible to modify the stereochemical outcome without modifying the structure of the phosphonate. They found greater (E)-stereoselectivity with the following conditions:
- Increasing steric bulk of the aldehyde
- Higher reaction temperatures (23 °C over −78 °C)
- Li > Na > K salts
In a separate study, it was found that bulky phosphonate and bulky electron-withdrawing groups enhance E-alkene selectivity.
Trisubstituted alkenes
[edit]The steric bulk of the phosphonate and electron-withdrawing groups plays a critical role in the reaction of α-branched phosphonates with aliphatic aldehydes.[17]
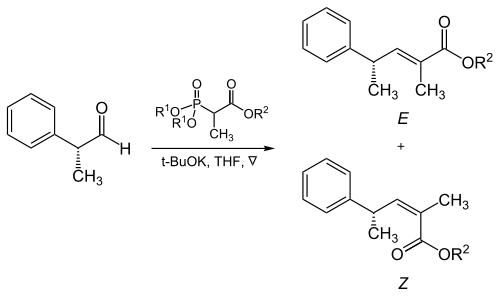
| R1 | R2 | Ratio of alkenes ( E : Z ) |
|---|---|---|
| Methyl | Methyl | 5 : 95 |
| Methyl | Ethyl | 10 : 90 |
| Ethyl | Ethyl | 40 : 60 |
| Isopropyl | Ethyl | 90 : 10 |
| Isopropyl | Isopropyl | 95 : 5 |
Aromatic aldehydes produce almost exclusively (E)-alkenes. In case (Z)-alkenes from aromatic aldehydes are needed, the Still–Gennari modification (see below) can be used.
Olefination of ketones
[edit]The stereoselectivity of the Horner–Wadsworth–Emmons reaction of ketones is poor to modest.
Variations
[edit]Base sensitive substrates
[edit]Since many substrates are not stable to sodium hydride, several procedures have been developed using milder bases. Masamune and Roush have developed mild conditions using lithium chloride and DBU.[18] Rathke extended this to lithium or magnesium halides with triethylamine.[19] Several other bases have been found effective.[20][21][22]
Gennari-Still modification
[edit]W. Clark Still and C. Gennari have developed conditions that give Z-alkenes with excellent stereoselectivity.[23][24] Using phosphonates with electron-withdrawing groups (trifluoroethyl[25]) together with strongly dissociating conditions (KHMDS and 18-crown-6 in THF) nearly exclusive Z-alkene production can be achieved.
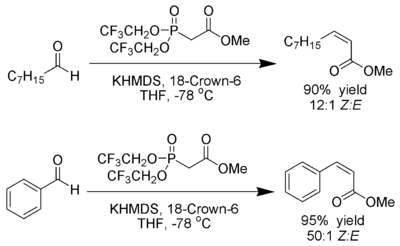
Ando has suggested that the use of electron-deficient phosphonates accelerates the elimination of the oxaphosphetane intermediates.[26]
See also
[edit]- Wittig reaction
- Michaelis–Arbuzov reaction
- Michaelis–Becker reaction
- Peterson reaction
- Tebbe olefination
References
[edit]- ^ Wadsworth, W. Org. React. 1977, 25, 73. doi:10.1002/0471264180.or025.02
- ^ Leopold Horner; Hoffmann, H. M. R.; Wippel, H. G. Ber. 1958, 91, 61–63.
- ^ Horner, L.; Hoffmann, H. M. R.; Wippel, H. G.; Klahre, G. Ber. 1959, 92, 2499–2505.
- ^ Wadsworth, W. S., Jr.; Emmons, W. D. J. Am. Chem. Soc. 1961, 83, 1733. (doi:10.1021/ja01468a042)
- ^ Wadsworth, W. S., Jr.; Emmons, W. D. Organic Syntheses, Coll. Vol. 5, p. 547 (1973); Vol. 45, p. 44 (1965). (Article)
- ^ Wadsworth, W. S., Jr. Org. React. 1977, 25, 73–253. (Review)
- ^ Boutagy, J.; Thomas, R. Chem. Rev. 1974, 74, 87–99. (Review, doi:10.1021/cr60287a005)
- ^ Kelly, S. E. Compr. Org. Synth. 1991, 1, 729–817. (Review)
- ^ B. E. Maryanoff; Reitz, A. B. Chem. Rev. 1989, 89, 863–927. (Review, doi:10.1021/cr00094a007)
- ^ Bisceglia, J. A., Orelli, L. R. Curr. Org. Chem. 2012, 16, 2206–2230 (Review)
- ^ Bisceglia, J. A., Orelli, L. R. Curr. Org. Chem. 2015, 19, 744–775 (Review)
- ^ Larsen, R. O.; Aksnes, G. Phosphorus Sulfur 1983, 15, 218–219.
- ^ Lefèbvre, G.; Seyden-Penne, J. J. Chem Soc., Chem. Commun. 1970, 1308–09.
- ^ Corey, E. J.; Kwiatkowski, G. T. J. Am. Chem. Soc. 1966, 88, 5654–56. (doi:10.1021/ja00975a057)
- ^ Reichwein, J. F.; Pagenkopf, B. L. J. Am. Chem. Soc. 2003, 125, 1821–24. (doi:10.1021/ja027658s)
- ^ Thompson, S. K.; Heathcock, C. H. J. Org. Chem. 1990, 55, 3386–88. (doi:10.1021/jo00297a076)
- ^ Nagaoka, H.; Kishi, Y. Tetrahedron 1981, 37, 3873–3888.
- ^ Blanchette, M. A.; Choy, W.; Davis, J. T.; Essenfeld, A. P.; Masamune, S.; Roush, W. R.; Sakai, T. Tetrahedron Letters 1984, 25, 2183–2186.
- ^ Rathke, M. W.; Nowak, M. J. Org. Chem. 1985, 50, 2624–2626. (doi:10.1021/jo00215a004)
- ^ Paterson, I.; Yeung, K.-S.; Smaill, J. B. Synlett 1993, 774.
- ^ Simoni, D.; Rossi, M.; Rondanin, R.; Mazzali, A.; Baruchello, R.; Malagutti, C.; Roberti, M.; Invidiata, F. P. Org. Letters 2000, 2, 3765–3768.
- ^ Blasdel, L. K.; Myers, A. G. Org. Letters 2005, 7, 4281–4283.
- ^ Still, W. C.; Gennari, C. Tetrahedron Letters 1983, 24, 4405–4408.
- ^ Janicki, Ignacy; Kiełbasiński, Piotr (2020). "Still–Gennari Olefination and its Applications in Organic Synthesis". Advanced Synthesis & Catalysis. 362 (13): 2552–2596. doi:10.1002/adsc.201901591. ISSN 1615-4169. S2CID 216228029.
- ^ Patois, C.; Savignac, P.; About-Jaudet, E.; Collignon, N. Organic Syntheses, Coll. Vol. 9, p. 88 (1998); Vol. 73, p. 152 (1996). (Article)
- ^ Ando, K. J. Org. Chem. 1997, 62, 1934–1939. (doi:10.1021/jo970057c)
