Wikipedia:Reference desk/Archives/Science/2016 September 24
| Science desk | ||
|---|---|---|
| < September 23 | << Aug | September | Oct >> | September 25 > |
| Welcome to the Wikipedia Science Reference Desk Archives |
|---|
| The page you are currently viewing is an archive page. While you can leave answers for any questions shown below, please ask new questions on one of the current reference desk pages. |
September 24
[edit]I'm trying to solve equation without the trick of taking mean speed during interval ε. But I see that if I take V(t+ε)=V(t)+εa(t) then the displasement increases xlsjpg. And when I take V(t+ε)=V(t)+εa(t+ε) then all is OK xlsjpg.
Feynman's trick also shows good results xls jpg.
So what should we actually do to solve ODE by numerical methods? What is correct way: to derive next step velocity from prev.step acceleration (V(t+ε)=V(t)+εa(t)), current step acceleration (V(t+ε)=V(t)+εa(t+ε)) or using trick V(t+0.5ε) = V(t-0.5ε)+εa(t)? The second case is simpler and shows as good results as 3rd.Username160611000000 (talk) 16:33, 24 September 2016 (UTC)
- You're applying Euler's method, which is known to yield the wrong results when applied to many simple equations. Even "trivial" ODEs can be unsuitable for numerical methods. The correct procedure is to study numerical integration so that you can have many methods to choose from, as well as other analytical tools and context to analyze when a method will work well. Nimur (talk) 16:09, 25 September 2016 (UTC)
- See also the Symplectic integrator article. Count Iblis (talk) 21:01, 25 September 2016 (UTC)
Vitamin C content on orange juice
[edit]How stable is vitamin C? Is its content much higher in freshly pressed OJ? — Preceding unsigned comment added by 31.4.151.67 (talk) 12:43, 24 September 2016 (UTC)
- Actually, it is usually much higher in packaged juice, but that is because vitamin C is added. EdChem (talk) 14:32, 24 September 2016 (UTC)
- Comparing the RDA amounts for an actual orange to the amount in cartons of juice - I don't reach that conclusion. SteveBaker (talk) 14:44, 24 September 2016 (UTC)
- Actually, it is usually much higher in packaged juice, but that is because vitamin C is added. EdChem (talk) 14:32, 24 September 2016 (UTC)
- For a carton of OJ (yeah, I know...not freshly pressed), you get 124mg of VitC from a 248g serving of juice. A typical orange yields 86g of juice - containing 43mg of VitC...so clearly it doesn't matter whether you're drinking juice from a carton or fresh pressed - you're still getting around 0.5mg of VitC per gram of juice.
- A vitC pill (let's go with Amazon's best seller "Natures Bounty") contains 1000mg of VitC - which is equivalent to around 8 servings of OJ. The bottle I have has a 2 year expiration date...and the law says that it still has to have 100% of the stated amount of the active ingredient present by that date. I did find a study that said that unopened sample of the vitamin C developed by Hoffmann-La Roche had maintained it's purity and potency for 20 years. So clearly the degradation rate is extremely slow if the vitamin is kept dry and away from the air.
- The RDA (recommended daily amount) for Vitamin C is about 60mg. So the juice of an orange and a half - or half a serving of OJ from a carton - or a tiny chip off of a pill should be just fine!
- Vitamin C is really ascorbic acid (aka "ascorbate") - which does slowly oxidize to dehydroascorbic acid which (according to our article) "...is actively imported into the endoplasmic reticulum of cells via glucose transporters. It is trapped therein by reduction back to ascorbate by glutathione and other thiols." another article says "On exposure to oxygen, ascorbic acid will undergo further oxidative decomposition to various products including diketogulonic acid, xylonic acid, threonic acid and oxalic acid."
- So it seems that Vit C does degrade by oxidation, albeit at a very slow rate. But even more than that, our bodies have adapted to this and can convert some of the decomposition products back into Vit C!
- Hence the question of how long the stuff lasts is a tough one because it depends on the form of delivery - how much oxygen and humidity is present when it's stored - and which specific decomposition path it goes through. Using expired VitC capsules should be OK because all that's happening is that the quantity of VitC present is slowly decreasing - and it would take a very long time for 1000mg to degrade all the way down to 60mg - which is the amount you actually need! One source I found said that a year after the expiration date printed on the packaging, 500mg if VitC had degraded to just 480mg.
- What I think we can conclusively say is that there is absolutely no benefit (from a Vitamin C perspective) to consuming fresh-pressed OJ compared to stuff that's been sitting in a carton on a supermarket shelf for a week or two. There are doubtless other reasons to consider consuming fresh-pressed OJ - but VitC degradation is absolutely not one of them!
- The shelf life of OJ is vastly less than the shelf-life of the vitamin C it contains...so don't worry about it.
- SteveBaker (talk) 14:44, 24 September 2016 (UTC)
What is the real definition of organic compounds?
[edit]According to Hebrew wikipedia is "compounds which contain carbon and hydrogen", but according to English wikipedia is a compound which contain Carbon. "An organic compound is any member of a large class of gaseous, liquid, or solid chemical compounds whose molecules contain carbon.". So I would like to know what is the real or right definition. 93.126.88.30 (talk) 14:20, 24 September 2016 (UTC)
- There is no single right answer: as the article organic compound states, any definition is "somewhat arbitrary". The Modern Classification section in that article gives as thorough an answer to your question as you're likely to get here. -- Ed (Edgar181) 14:25, 24 September 2016 (UTC)
- All of the definitions have some problems. According to Hebrew Wikipedia, CCl4 is not organic, but CHCl3 is, which I think is a bit silly. Also, mellitic acid (a benzene ring with six carboxylic acid groups dangling off it) is not organic by the definition that requires C–H bonds, although it certainly looks like it should be! But according to the English Wikipedia definition (without the later clarification that you've cut off), CO2 and SiC are organic, which I don't think anyone agrees with. There is no simple definition that will correspond to the vague mental image we have in our minds of what is "organic" and what isn't. Double sharp (talk) 03:05, 27 September 2016 (UTC)
Find article for fuel gas transported through plumbing
[edit]Urban areas often have a system where fuel gas (short hydrocarbons) are transported to many homes and other buildings from a central location through plumbing (long pipes), and in the buildings it is used for heating or cooking. I'd like to find the article on this Wikipedia specifically on this system, in contrast to eg. Liquefied petroleum gas (fuel gas transported to homes in cylinders carried by vehicles). I tried to search and follow links, but can't find it. Maybe I'm dumb, but I can't find such an article. If there is no such article, then I'd like to know what terms are usually used in English to describe this system. – b_jonas 16:21, 24 September 2016 (UTC)
- History of manufactured gas is the relevant article. The usual term in BrE is "gas main" or "gas pipe", both of which redirect to Pipeline transport. Tevildo (talk) 16:58, 24 September 2016 (UTC)
- You will find most of what you want in the Natural Gas article. Wymspen (talk) 16:57, 24 September 2016 (UTC)
- (Multiple EC) it probably should be covered in Pipeline transport however this mostly concentrates on long range transport including in the section on oil and natural gas. However "Distribution pipelines" are mentioned later on. There is also the somewhat weird Central gas system. Nil Einne (talk) 17:31, 24 September 2016 (UTC)
- That last article linked by Nil Einne might be a candidate for Deletion if no-one can significantly improve it. Currently its one Reference links to a no-longer-extant web page, and there is nothing in the article to indicate that it's anything more than a theoretical concept with no real-world examples. {The poster formerly known as 87.81.230.195} 90.202.211.191 (talk) 17:41, 24 September 2016 (UTC)
- Lack of reliable sources may be an issue, but there's no ban on articles on theoretical concepts. See Project Daedalus, for example. StuRat (talk) 17:55, 24 September 2016 (UTC)
- A search on "central gas system" comes up with lots of pages like this, which describe the supply of gas to a site from a central tank - the opposite to the system described in the current article. It's probably possible to put together a new article about such installations, but it does seem as though the existing article needs to be changed. Tevildo (talk) 18:30, 24 September 2016 (UTC)
- Note that the article in question appears to have been created by someone with a WP:COI [2] Nil Einne (talk) 19:46, 24 September 2016 (UTC)
- That's true - I've therefore PRODded it. It is, of course, still open to anyone to salvage or recreate it. Tevildo (talk) 14:14, 25 September 2016 (UTC)
- Note that the article in question appears to have been created by someone with a WP:COI [2] Nil Einne (talk) 19:46, 24 September 2016 (UTC)
- Thank you all. – b_jonas 13:26, 25 September 2016 (UTC)
Diseases that attack fungi
[edit]We all hear about disease caused by fungi in humans and other organisms. However, are there any specific diseases that afflict fungi like mushrooms? Do any bacteria or viral phages attack fungal cells? 74.71.135.72 (talk) 16:33, 24 September 2016 (UTC)
- Mycovirus is the name for viruses that infect fungi. There has also been some study of bacteria that infect fungi especially cultivated mushrooms, e.g. Pseudomonas fluorescens and several other Pseudomonas [3]. This source [4] seems to have a decent but brief overview. Note the list of parasites that affect fungi include fungi. Nil Einne (talk) 17:52, 24 September 2016 (UTC)
- I notice that the apparently parallel term Mycobacterium is actually used to designate bacteria that infect mammals (etc?), not fungi, being so named merely because they may grow on culture mediums in a way resembling fungi. What – instead – are fungi-infesting bacteria called collectively? [I see that Nil Einne's link 28 uses "Mycopathogenic bacteria".] {The poster formerly known as 87.81.230.195} 90.202.211.191 (talk) 17:56, 24 September 2016 (UTC)
- Note that bacteria which infect fungi may not infect fungi only, so may not be named after fungi in any way. StuRat (talk) 18:02, 24 September 2016 (UTC)
- The Serbian source uses mycopathogenic bacteria however the book sources demonstrates it can get complicated what you want to include in this list given the variety of possible effects, e.g. antibiotics that affect fungi, lytic enzymes directed as fungal cell was degradation and so how you define the relationship between the bacteria and fungi. Nil Einne (talk) 18:13, 24 September 2016 (UTC)
- (EC) Note the list of parasites that affect fungi include fungi, and these have also been studied both because of their effect on cultivated mushrooms but also apparently for biocontrol of fungi that affect cultivated plans e.g. [5] (if you do a research or even general search you should find lots of these). Mycoparasites appears to be the general term for parasites that affect fungi although it seems to be most commonly used in reference to fungi that affect fungi although that's probably because they appear to be of most interest to biocontrol. And biocontrol of fungal diseases of plants is of far greater interest that diseases affecting cultivated mushrooms given the relative sizes of the markets. Nil Einne (talk) 18:13, 24 September 2016 (UTC)
- If I collect and eat mushrooms, would that qualify as me being a mushroom disease? Count Iblis (talk) 21:06, 24 September 2016 (UTC)
- No, you would be a mushroom predator and all around fun guy. StuRat (talk) 22:48, 24 September 2016 (UTC)
What is the basic structure of all amino acids?
[edit]Do all of the amino acids have the same structure of H2NCHRCOOH as it's illustrated in the picture that attached? I've read the article but I just want to ensure that I understood the answer for my question. (By the way is the letter R in the illustration symbolizes the right side? (if so, why only right side rather than left)
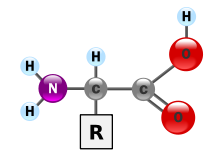
93.126.88.30 (talk) 17:24, 24 September 2016 (UTC)
- Answers: 1. No, not all amino acids have that structure. An amino acid has both an acid group and an amino group, but they don't have to be connected through just one carbon atom. Other examples include p-aminobenzoic acid and GABA γ-aminobutyric acid. Even amongst the 20 naturally occurring amino acids used to make proteins, not all have a primary amine group (NH2). For example, look at
tryptophanproline.Correcting the error noted below by Adrian J. Hunter.
- 2. The R does not refer to right but to the side chain. In glycine, the R group is an H atom, R = H. In alanine, the R group is a methyl group, R = CH3. Each naturally occurring amino acid has a different R group. EdChem (talk) 17:53, 24 September 2016 (UTC)
- Very often, when people say "amino acids" they're really referring to the 20 proteinogenic amino acids – the 20 amino acids that are commonly used by cells to make proteins. The diagram you've provided illustrates the typical structure of these proteinogenic amino acids. The only one of the 20 that does not quite conform to this structure is proline.
- EdChem, my chemistry is rusty, but doesn't tryptophan have a primary amine group? I thought proline was the only proteinogenic amino acid that does not. Adrian J. Hunter(talk•contribs) 08:16, 25 September 2016 (UTC)
- Adrian J. Hunter thanks for the ping. You are absolutely correct, tryptophan has a primary amine group and it is proline with the secondary amine structure. I was thinking of proline, and have no idea why I typed tryptophan. I have corrected my error above and noted you pointed it out. :) EdChem (talk) 12:54, 25 September 2016 (UTC)
- There are a number of assumed caveats when we speak of "amino acids". The most important is that yes, we're usually thinking of amino acids with a single alpha carbon that connects the COOH and NH2, which is an important feature for polypeptide structure. Proline is an outlier in being a secondary rather than a primary amine, i.e. an "imino acid".[6] This variation in structure is very important for its role in the organism; it disrupts regular helix structure at designated points, allowing particular parts of proteins to be more accessible or to make turns at desired places, and there is a specific enzyme prolyl isomerase that is needed in order for it not to lock proteins into undesired conformations. I should note that our article recognizes selenocysteine as proteinogenic for a total of 21, even though its incorporation at a nominal stop codon can be somewhat inconsistent; we're not as consistent in recognizing the bacterial pyrrolysine, though in amino acid we actually have a claim of 23, not backed by sources... I should look into that but first I'd prefer to make sure nobody discovered the 23rd while I wasn't looking; I'm sure it's out there... There are also a number of non-proteinogenic amino acids that would often be considered "amino acids" in a narrower sense of things that can be isolated by hydrolyzing a protein, such as hydroxyproline and allysine; these are not added to proteins during synthesis but modified after the protein is made. And then there are lots of other primary amino acids with NH2 and COOH on an alpha carbon like L-DOPA that could go into a protein except biology doesn't want it to (in the case of that one, I think it's too reactive ... I wouldn't let that thing near a ribosome either with two OHs on a phenyl ring! That's expensive equipment there...) We make up these categories for a reason, but since our reasons differ sometimes we have to be very careful which category we're looking at. Wnt (talk) 16:36, 25 September 2016 (UTC)
- @Wnt: that NCBI reference is wrong, proline is most definitely not an imino acid, though it can be enzymatically converted to the imino acid (S)-Δ1-pyrroline-5-carboxylic acid by pyrroline-5-carboxylate reductase. An imino acid requires an imine functionality, which proline lacks. The IUPAC Gold Book characterises the term as "obsolescent" for cases like proline, so I accept it was used historically (as our amino acid article recognises) but it is (formally) incorrect and I would avoid it. Further, the 20 standard protein amino acids are a subset of the broader term which definitely does include not only the others you mentioned but also GABA and PABA and many others. As for the claim of 23 in the standard set for protein synthesis, I have seen 22 quoted regularly (the regular 20 plus selenocysteine and pyrrolysine, as you mentioned), but don't recall seeing a claim of 23. EdChem (talk) 04:03, 26 September 2016 (UTC)
- @EdChem: Thanks! I have seen the "imino acid" thing so many times I didn't think it could be wrong, even though the term never made a whole hell of a lot of sense. "Secondary amino acid" is more than fine as a replacement term. Wnt (talk) 15:28, 26 September 2016 (UTC)
- @Wnt: that NCBI reference is wrong, proline is most definitely not an imino acid, though it can be enzymatically converted to the imino acid (S)-Δ1-pyrroline-5-carboxylic acid by pyrroline-5-carboxylate reductase. An imino acid requires an imine functionality, which proline lacks. The IUPAC Gold Book characterises the term as "obsolescent" for cases like proline, so I accept it was used historically (as our amino acid article recognises) but it is (formally) incorrect and I would avoid it. Further, the 20 standard protein amino acids are a subset of the broader term which definitely does include not only the others you mentioned but also GABA and PABA and many others. As for the claim of 23 in the standard set for protein synthesis, I have seen 22 quoted regularly (the regular 20 plus selenocysteine and pyrrolysine, as you mentioned), but don't recall seeing a claim of 23. EdChem (talk) 04:03, 26 September 2016 (UTC)
- @Wnt: Regarding #23 (the 20 obvious longterm standards, plus Sec and Pyl), N-formylmethionine (fMet) seems to sometimes/sort-of be considered. Our proteinogenic amino acid and amino acid articles keep wavering on the inclusion of it and their talkpages seem inconclusive. DMacks (talk) 04:36, 26 September 2016 (UTC)
- I usually think of there as being 21: the 20 standards plus Sec. This is because these are the only ones that are actually coded in by eukaryotes. (Also, Sec has a somewhat different chemistry from Cys, since selenols are better nucleophiles. At biological pH, the thiol on Cys keeps its proton if it hasn't been oxidised away, but the selenol on Sec is acidic enough to be deprotonated already.) Double sharp (talk) 12:10, 27 September 2016 (UTC)
- @Wnt: Regarding #23 (the 20 obvious longterm standards, plus Sec and Pyl), N-formylmethionine (fMet) seems to sometimes/sort-of be considered. Our proteinogenic amino acid and amino acid articles keep wavering on the inclusion of it and their talkpages seem inconclusive. DMacks (talk) 04:36, 26 September 2016 (UTC)
Tying up a horse in old TV shows and movies
[edit]They often just loop the reins once or twice around a post and call it good. The horse would easily be able to get loose. So, did/do people really tie knots around the post, or does the horse just follow the "honor system" and stay put, even if it could easily escape ? StuRat (talk) 18:35, 24 September 2016 (UTC)
- The old movies showed short-term actions, the horse was not even unsaddled, it was sort of like parking your car on the street by the store while you shopped (or went to the bar and drank). If they tied reins to a post and the horse spooked and set back on the line, the horse would just break the reins anyway because they were thin leather and the bit would hurt their mouths in the process. The loop system (usually twice around in the real world) put some restraint on the horse (who would not be inclined to leave, as working horses learn to take their rest when they can get it; plus, there often were other horses there) but if all hell broke loose (i.e. fire, bar fight), the horse could get away without breaking equipment (still might step on one rein and break it later, but that's a different problem). If they were going to keep the horse tied for a long time, like overnight, they'd unsaddle and put on a halter and tie with a lead rope. Montanabw(talk) 20:08, 24 September 2016 (UTC)
- Another reason they were tied that way is because real cowboys didn't go around carrying halters and lead ropes; they'd snag on brush and cause too many problems. From what I understand, it was hard enough to carry a lariat without it hanging up on stuff and potentially hurting the cowboy. Most Western horses used on ranches were taught to ground tie anyway. The horse would stand completely still if the ends of the reins were dropped on the ground, and remain there until the rider came back for it. That was particularly useful in places where there were no trees or anything to tie a horse to. White Arabian Filly Neigh 21:52, 24 September 2016 (UTC)
- I'm amazed that horses can be so well-trained. Few dogs could be trained to that degree. StuRat (talk) 22:21, 24 September 2016 (UTC)
- They will wander off eventually if they are completely ignored. Ground tying worked great for stuff like fixing fence or doctoring a calf. Not so good for eating lunch or drinking at the bar. Best images of how the real thing worked are probably paintings of C.M. Russell, he lived the life and was dedicated to authenticity. Montanabw(talk) 22:44, 24 September 2016 (UTC)
- I am speculating here. My experience with dogs is that many of them will "sit and stay", so long as the owner remains in sight. Is this the same with horses and ground tying? DrChrissy (talk) 23:53, 24 September 2016 (UTC)
- Pretty much. They do think for themselves. Also, horses are inherently a little bit lazy when given the opportunity, so they'd stay tied to the hitching post and not fight a minimal attachment because they could hang with their buddies and sleep! Montanabw(talk) 03:51, 25 September 2016 (UTC)
- That has reminded me of an observation. In all those old movies I have never see anyone hitching up his horse and having a kid approaching offering to “mind his 'orse”. Horses back then (relative to a cheap Japanese cars today) cost a lot to fuel and maintain. Some US states hung horse thieves with-out trial. The saddle was the equivalent of a modern-day-lap-top-sitting-on-the-back-seat-just-there-for-the-taking. Yet, the cowboy and western films keep showing horse riders etc. hitching up their horses out side a saloon as if they expect their steeds, saddles and Winchester rifle, will still be there when they return. There were no food stamps back in those days, it was either risking the noose or starvation. So where are all those kids that horse minded? – too bothersome for the film companies to hire perhaps. Also, horses are very strong animals. If the reins don't break, the hitching post will, and as they are pulling, the bit has no effect.--Aspro (talk) 21:57, 24 September 2016 (UTC)
- One advantage of snowmobiles is that it doesn't turn into rotting meat & poop if you forget its weekly 15 gallons of fuel one August. Sagittarian Milky Way (talk) 23:05, 24 September 2016 (UTC)
- How is this possibly furthering the discussion - I was half tempted to delete the post. DrChrissy (talk) 23:48, 24 September 2016 (UTC)
- Horses will die from thirst in storage and leave a half ton of rotten meat etc. in your garage while internal combustion devices will not. They need many gallons of fuel (hay and water) to prevent this. Maybe it was a stupid joke. Sagittarian Milky Way (talk) 00:07, 25 September 2016 (UTC)
- Horses do have the advantage that those able to put in the substantial work involved can (on average) count on getting new ones for free. I'm not sure I'd count them out just yet. I'm seeing mention of horses, at least momentarily, cracking 55 miles an hour; no matter how slowly ordinary breeding [7] may improve them, I would not rule out more significant improvements from genetic engineering when it is done at a level of sophistication not currently imaginable. So I wouldn't rule out the day when horses that require much less maintenance and which can reach higher speeds with greater burdens can actually challenge the traditional automotive industry - especially if sinister trends like the tracking and I assume eventually restriction of what can be done with self-driving cars become extreme enough to drive a search for loopholes. Wnt (talk) 17:17, 25 September 2016 (UTC)
- I'm rather skeptical. Genetic engineering can't overcome the inherent limitations of biological systems. Even if you could get a horse to go faster than that, it would die, along with any passengers, if it fell at such speeds, making it an extremely dangerous form of transportation. StuRat (talk) 17:43, 26 September 2016 (UTC)
- Aspro, I'm a little unconvinced that the bit would have no effect. That might be true if the horse was backing up in a straight line, but if the horse was reacting to something that spooked it, their head would also be moving side to side and so the bit would likely cause damage. DrChrissy (talk) 22:05, 24 September 2016 (UTC)
- This behaviour doesn't need the animal to consciously evaluative the situation. Bits don't control by producing pain but by exerting pressure which the animal quickly comes to understand. If the animal gets spooked and finds its natural response produces pain from the bit... doesn't it naturally stops witching its nose this way and that and just move forwards or backwards? You can see this same behaviour in little children that get confronted by two bullies. Second response (after witching) is to either try to forces their way through them rapidly or draw away rapidly. Even a <14 hands pony can break a hitching post like match-wood. Result: kid doesn’t receive any punches in his escape and 'orse doesn’t doesn't get injured either. However, you may well have come across equines with mouth injuries. What were the exact circumstances (if that doesn’t break patient confidentiality)? The OP is asking about the likes of Roy Rogers hitching up in films. Montanabw below talks about 'modern' nylon reins, 'modern' unbreakable hitching posts, and curb bits that the cow drivers used ( the modern day equivalent of the Ford Mustangs and Toyota pick-up trucks). Back in the day of the Wild West owners put their horse first and feed and watered them first after a long ride because their livelihood depended upon that animal. Also, if a driver (cowboy) did hitch up in front of a saloon with a curb bitted animal, isn't that all the more reason to hire a horse minder? No nylon reins, no unbreakable hitching posts in those days. For the film buffs out there. Have you ever seen horses being hitched with curb bits? Rowdy Yates (Clint Eastwood) in Rawhide (TV series) was a driver. Yet has anybody out there spotted a curb bit? One doesn't just get a horse shod and expect 20,000 miles out of it. It need to be re-shod about every three months. Also horses do not even achieve 40 miles to the gallon every day. One can just to forget to wash the car for a few weeks but if your livelihood depends on a horse, it gets groomed regularly and any hitching post provided by a general store, saloon or whatever that is not suitable causes the owner to lose custom. The OP is asking about horses as depicted in films.--Aspro (talk) 00:41, 25 September 2016 (UTC)
- Aspro, you've never seen a horse tied hard and fast set back and hit a well-set post; it's damn scary. And while a cheap rail might break if tied with a solid rope, only an idiot ties a horse to a flimsy object. (Idiocy happens, but more often, the rail or post comes loose and the horse drags it off at a dead run). Usually, a horse sets back but gives up quickly if they aren't thrown into a worse panic by pain. If they fight the rope, more often the rope or the halter breaks. Montanabw(talk) 03:51, 25 September 2016 (UTC)
- First off, reins are single-thickness pieces of strap leather, no more than about 3/4 of an inch across and at the most maybe 1/8' to 1/4" thick. Even a 500-lb pony can break them. But, the bit is in the mouth, which is sensitive, so even breaking reins hurts like heck and that actually increases the panic of the horse. I was chatting at my talk that I've seen people tie horses up with a bridle with nylon reins (modern material, doesn't break easily) and set back, resulting in their tongues being injured. Even if a horse sets straight back, a curb bit in particular will do some nasty things. The hitching post usually will NOT break... not if properly constructed. Montanabw(talk) 22:44, 24 September 2016 (UTC)
- When I was learning to ride, I seem to remember that there were tying hoops embedded in concrete walls of the stables. However, there was a loop of string (not rope) attached to this which the reins or halter were tied to. Obviously this string was the weak link in the chain and the intention was this would snap before the horse was (seriously) injured. DrChrissy (talk) 19:40, 25 September 2016 (UTC)
- Whilst the films obviously have a lot of poetic licence, we were told when I was a young Scout that they're based on a highwayman's hitch.--Phil Holmes (talk) 10:53, 25 September 2016 (UTC)
- On the bits, yes, a curb bit was what they used back then. A snaffle bit was seen as fit only for young untrained horses, plow horses, or carriage horses. Cowboys also rode one-handed most of the time, and the curb bit makes that easier, as a snaffles have the tendency to collapse in the middle when the rein is lifted (which is what you do to ride one-handed, lift the rein and touch it to the horse's neck in the way you want it to go, as opposed to pulling the horse's head around in two-handed riding.) White Arabian Filly Neigh 21:11, 25 September 2016 (UTC)
- So as not to confuse. Have just watch a few clips of Rawhide (TV series) etc. (on youtube), where the main cast were all competent horsemen. They do appear to be using curbs -when out on the range. As you know Lassie was not one dog but several nearly identical trained collies. Each trained to excel for different scenarios. Same with horses and actors. Actors who may be not competent horsemen (which probably applies to most) in close-up just sit on their steeds whist delivering their lines. When they are galloping after the bad-guy, its always long-shots with a stunt-double in the saddle ( this is often insisted upon buy the insurance underwriter if it is a famous actor.) So the hitching up shots and close ups are often done with a trained horse with a snaffle. Hope I haven't destroyed anyone's delusions here about film reality vis. real life and the OP's question was about films (or as its about film should that be illusions). Also, has anyone noticed that every time cowboys, etc, are sitting around a camp-fire, there is always a wooden barrel there. Why...? Yes, you've guessed it. The hourly cost of filming is so high that they can't waist time kindling a fire and maintain continuity (fiction) over many takes and retakes. The fire has to appear the same all the time. Therefore, the barrel is were they hide the gas cylinder which fuels the fire and the wood on the fire isn't real wood either. --Aspro (talk) 20:00, 26 September 2016 (UTC)
- This reminds me of some morning talk show (The Today Show ?) where their "expert" said that the proper way to start a fire in the fireplace was to put the large logs at the bottom, for stability, and the kindling on top. Needless to say, nothing happened, until they came back from commercial and had a blazing fire. I tried to spot the empty gasoline can nearby. :-) StuRat (talk) 00:12, 27 September 2016 (UTC)
- Yes, from what you say, a little time-compression has taken place during the com-break. Also, it should have not been 'blazing'. The whole idea (as you have probably gathered) is to have a slow burning fire that provides hot embers on which to cook upon -for an extended period. The top layer of ash tends to suppress flames. Yet, on TV that may have not looked impressive enough– so it may have been doctored to show lots of flames, maybe with bottled gas. Right way a building a fire though -for cooking purposes- (and to keep the log-cabin warm during the day whilst one is out hunting sasquatch). Can't fault the expert for that.--Aspro (talk) 14:21, 27 September 2016 (UTC)
- If you want to start a wood fire without using an accelerant, you need to put the kindling below the larger branches and then logs, as heat rises. It you put it on top, the logs won't catch fire. (You could build something akin to a tiny log cabin, filled with kindling, and get the roof to catch fire that way, then the sides should catch fire from there.) StuRat (talk) 15:40, 27 September 2016 (UTC)
- No, you're wrong, you absolutely can start a nice fire in a fireplace with large logs on the bottom and kindling on top. Or maybe 'you' can't, but I can ;) A casual google search of "upside down fire" will get you plenty of demonstrations. In my experience, it works better in fireplaces or stoves, compared to outdoors. SemanticMantis (talk) 16:40, 28 September 2016 (UTC)
- It may depend on the wood you use. I always brought wood in right from the woodpile outside, so it was slightly damp. I tried bringing wood in first to sit next to the fireplace to dry out, but was disgusted at all the bugs and spiders that crawled out of the wood, so resolved to toss them directly into the fire when I bring them in, to kill all the critters. StuRat (talk) 18:02, 28 September 2016 (UTC)
- I am of a that certain age and live in a certain country (UK) where I/we burnt solid fuel for heating and cooking on kitchen-range until about the 1960's. We did 'not' need to use 'any' accelerants...this explains the technique. > http://www.google.co.uk/url?q=http://scouts.org.uk/media/581688/Victorinox-Survival-Skills-Resource-Building-a-Fire_Final-Proof.pdf&sa=U&ved=0ahUKEwjE4KKo-rHPAhWErRoKHYPXCBwQFggUMAA&usg=AFQjCNFbpTGhCGMCL0NTb8IreIMX-uIoKg < To do otherwise, would have created a too hotter fire, over heated the room at times, require constant maintenance and made the cold drafts into the room even worse. So (IMHO) the 'expert' is correct - as this is the right way to build a fire. User:DrChrissy resides in the West Country (well, west of me anyway) and may visit farm houses which still today have log-fires burning this way. Disregarding the possible carcinogenic properties, a log-fire, adds a very homely aroma to the room, according to the type of wood being burnt and its mix of terpenoids, oleoresins (such as Cinnamic acid (i.e., a cinnimon smell), etc., etc., et cetera.. Don't criticise you at all... for being wisely wary of adverts. After all, a com-break is too shorter time to get such a fire started properly. The crux of the matter is on the correct way to build a fire and the 'expert' had it right - this once.Aspro (talk) 13:12, 28 September 2016 (UTC)
- If you mean starting a fire in a wood stove, that's a bit different, as they are designed to retain heat, while an open fire like this one allows the heat to rise and leave the area immediately, unless that fire is below what you are trying to ignite. StuRat (talk) 14:55, 28 September 2016 (UTC)
- Did the advert feature a wood stove? I didn’t say anything about a stove – so why should I mean it – if so, I would have said. What you depicted as a wood stove is like what we think of as an AGA cooker. You go on to say “while an open fire like this one” (which one) (?) leaving us to mind-read you. Oh, come on Stu – sit back, relax and focus like you could before.--Aspro (talk) 15:47, 28 September 2016 (UTC)
- @Aspro. You are correct about me living in the west country. I actually live reasonably close to the Somerset levels which contain a number of large, ancient peat bogs. Locals sometimes burn the peat in their fires (although I suspect this is illegal). DrChrissy (talk) 17:36, 28 September 2016 (UTC)
- @ User:DrChrissy As far as I know it is not illegal, unless the peat come a site of Special Scientific Interest (whether this is right or wrong morally/ecologically - is another issue). There are acres and acres of peat, many feet thick out west. I once (twice, thrice or more times) – (Oh, the West Country cider is so strong there, that I can't remember anything clearly other than the barmaids names) stopped at an Inn on Dartmoor that kept its peat fire going continuously (and one can see that that by the soot), (peat-fire produce a lot of shoot). All because in Warren House Inn, a peat fire has been burning since 1845 because a witch (Vixiana – can't forget her name either ) was banished into the fire some 400 hundred years earlier (in the old pub that stood near by) and the embers transfered to the new Pub and whilst the fire stays burning there -she still can't escape.--Aspro (talk) 19:03, 28 September 2016 (UTC)
- What a great story! I live on the edge of one of the THatcher's orchards so I know all about the local apple juice - both the pleasure and pain! DrChrissy (talk) 19:14, 28 September 2016 (UTC)
- @ User:DrChrissy As far as I know it is not illegal, unless the peat come a site of Special Scientific Interest (whether this is right or wrong morally/ecologically - is another issue). There are acres and acres of peat, many feet thick out west. I once (twice, thrice or more times) – (Oh, the West Country cider is so strong there, that I can't remember anything clearly other than the barmaids names) stopped at an Inn on Dartmoor that kept its peat fire going continuously (and one can see that that by the soot), (peat-fire produce a lot of shoot). All because in Warren House Inn, a peat fire has been burning since 1845 because a witch (Vixiana – can't forget her name either ) was banished into the fire some 400 hundred years earlier (in the old pub that stood near by) and the embers transfered to the new Pub and whilst the fire stays burning there -she still can't escape.--Aspro (talk) 19:03, 28 September 2016 (UTC)
- The pain only arrives if you forget to take a jar of the stuff home to drink pro re nata during the night and following morning. It is effective as the hair of the dog (whatever that might be). You now been both advised and warned regarding 'apple juice' from this part of the British Isles.--Aspro (talk) 20:33, 28 September 2016 (UTC)
- Does the peat burn better when it has a body in it ? :-) StuRat (talk) 17:57, 28 September 2016 (UTC)
- What do you call a man who has been buried for 60,000 years. Answer: Pete
- Lets give him his full name Pete Marsh--Aspro (talk) 19:54, 28 September 2016 (UTC)
- And in Russia, Clay Bogdanovich. Akld guy (talk) 20:54, 28 September 2016 (UTC)
- Lets give him his full name Pete Marsh--Aspro (talk) 19:54, 28 September 2016 (UTC)
- What do you call a man who has been buried for 60,000 years. Answer: Pete
- Does the peat burn better when it has a body in it ? :-) StuRat (talk) 17:57, 28 September 2016 (UTC)
- I started by saying "proper way to start a fire in the fireplace". It was a rather open fireplace on the show, so very little heat would be reflected back. StuRat (talk) 17:57, 28 September 2016 (UTC)
- It has only been in resent decades (within living memory – my living memory) that people accepted that during winter they could not just wear short leaved shirts during winter. The solid fuel fire just kept off the chill. Today, we all have automatic central heating systems that come-on when the temperature drops. Fuel to-day is unbelievable cheap. Back then is wasn’t. --Aspro (talk) 19:45, 28 September 2016 (UTC)
- "resent" decades sound very retro. :-) StuRat (talk) 21:49, 28 September 2016 (UTC)

