Wikipedia:Reference desk/Archives/Science/2011 February 13
| Science desk | ||
|---|---|---|
| < February 12 | << Jan | February | Mar >> | February 14 > |
| Welcome to the Wikipedia Science Reference Desk Archives |
|---|
| The page you are currently viewing is an archive page. While you can leave answers for any questions shown below, please ask new questions on one of the current reference desk pages. |
February 13
[edit]Hill, James B. (1945). Autobiography
[edit]An article references "Hill, James B. (1945). Autobiography. Raceland, Louisiana, USA: James B. Hill. pp. 200". How can I get more information on this autobiography? —Preceding unsigned comment added by 66.97.56.130 (talk) 01:34, 13 February 2011 (UTC)
- You could try your local library; they're unlikely to have a copy themselve, but they do they have experts in locating books. But it is not certain that it is findable at all. The format of your reference indicates that the book is self-published (the name that comes after the place of publication is that of the publisher), so it may be a small run that has never been catalogued by a general library. The book does not seem to exist in the Library of Congress's on-line catalog. –Henning Makholm (talk) 02:52, 13 February 2011 (UTC)
- Also, this would be better asked on the humanities desk. –Henning Makholm (talk) 02:52, 13 February 2011 (UTC)
Time when Sun is highest in the sky
[edit]On any given day, is the Sun at its highest in the sky at exactly the same time in all places with the same longitude? (By "same time" I mean same time as measured by a single reference clock. Ignore any issues to do with local time.) I've always assumed, without thinking too much about it, that the answer was "yes", but now I'm beginning to doubt it. 86.183.3.100 (talk) 04:02, 13 February 2011 (UTC)
- Why are you doubting it? It seems right to me. Dauto (talk) 04:40, 13 February 2011 (UTC)
- Don't doubt it. I've never heard or thought otherwise. HiLo48 (talk) 04:46, 13 February 2011 (UTC)
- Yes, local noon has to happen simultaneously on an entire meridian. Noon happens at the moment the abstract plane that contains the Earth's axis and the place in question sweeps through the center of the Sun. But this plane is the same for all places on the meridian, so their noons are the same.
- (I'm assuming you're not interested in things like relativistic aberration of the sunlight due to the different rotational speeds on points at different distances from the axis. Not sure the effect is measurable anyway). –Henning Makholm (talk) 04:49, 13 February 2011 (UTC)
- One possible exception is in the Arctic or Antarctic, where, depending on the time of year, the Sun may not come up at all that day. So, if there is no Sun at all, the time when it is highest in the sky is undefined (unless, I suppose, you consider that to be when the Sun is closest to, but still below, the horizon). StuRat (talk) 04:10, 14 February 2011 (UTC)
Thanks for the replies. I am trying to calculate the declination of the Sun as seen from Earth at different latitudes, and at different times of year, using a simplified model. The model assumes that the Earth’s orbit is circular and ignores various other complications such as precession. I know that the model is not exact, but it does not seem feasible that making it exact by adding ellipticity and the other tweaks will exactly eliminate the discrepancy that I observe. In the model:
k is number of Earth rotations per year, which I’m assuming is 366.25
alpha is angle of Earth’s tilt, which I’m assuming is 23.5 deg.
a is the time at which we measure the Sun’s declination, in years. a = 0 is the moment of the December solstice in some year (for me it doesn’t matter which one). a = 1 is the moment of the December solstice one year later.
phi is the latitude on Earth from which we observe the Sun
I assume that the point from which we observe the Sun is on the longitude that is exactly facing away from the Sun (i.e. midnight) at a = 0.
I set up a coordinate system whereby the x and y axes lie in the plane of the Earth’s orbit, with the x-axis pointing in the direction from the Sun to the Earth at a = 0, and the y-axis pointing in the direction that the Earth is (instantaneously) moving in its orbit at a = 0. The z-axis points North out of the orbital plane.
To find the declination of the Sun, I first calculate the direction (unit) vector (x,y,z) from the centre of the Earth to the viewpoint on the Earth’s surface at time = a. I do this as follows:
At a = 0, without yet accounting for the Earth’s tilt, we know (x,y,z) = (cos(phi), 0, sin(phi)).
Then, I account for the Earth’s rotation at time = a by rotating vector (x,y,z) about the z-axis (anticlockwise as looking from positive z to negative z) by an angle 2*pi*k*a.
Then I account for the axial tilt by rotating (x,y,z) about the y-axis (clockwise as looking from negative y to positive y) through angle alpha.
Then I calculate theta, the angle between the resulting vector (x,y,z) and the vector from the centre of the Earth to the Sun at time = a, which I make to be (-cos(2*pi*a), -sin(2*pi*a), 0).
Finally I calculate: Sun’s declination = pi/2 - theta
According to my calculations, this results in:
declination = -pi / 2 + acs(cos(alpha)*cos(phi)*cos(2*pi*a)*cos(2*pi*k*a) + cos(phi)*sin(2*pi*a)*sin(2*pi*k*a) + sin(alpha)*sin(phi)*cos(2*pi*a))
The trouble is that I can’t see either numerically or analytically how the maxima of this (w.r.t a) are independent of phi. I get very slight variations.
What am I doing wrong? Thanks to anyone who has got this far! 81.159.104.144 (talk) 14:35, 13 February 2011 (UTC)
- Hmmm, actually you are right and we were wrong. I get the same equation as you do.
- What went wrong in our intuitive understanding was that we confused "Sun highest in the sky" with "Sun appears due south". They are not exactly equivalent. The "due south" moment (which is simultaneous along an entire meridian) is when the fixed star that the Sun is momentarily in front of has its highest elevation. At that time, this star moves horizontally, but at the same time the Sun is moving along the ecliptic relative to the fixed stars, and this movement generally has a north-south component. So at the "due south" moment, the Sun is actually still moving slightly upwards in the sky, or already moving downwards, depending on which hemisphere we are in, meaning that "highest solar elevation" noon does vary very slightly along the meridian.
- This also helps clear up the apparent discontinuity at the poles: When we move closer to the pole, in the limit they daily variation in the Sun's elevation gets much smaller than the variation due to the ecliptic movement. So on any meridian, the "highest elevation" moment during the summer solstice day must converge towards the exact solstice moment as the latitude approaches 90° exactly. –Henning Makholm (talk) 17:50, 13 February 2011 (UTC)
- Hi Henning, thank you very much for taking the time to look at this. I'm delighted that you now get the same result as me; staring at these equations has been driving me nuts! 86.179.118.226 (talk) 18:44, 13 February 2011 (UTC)
- Henning Makholm wrote" "The "due south" moment (which is simultaneous along an entire meridian)" You are forgetting about the part of the earth south of the Tropic of Capricorn where the noon sun is due north. Between the Tropic of Capricorn and the Tropic of Cancer the sun may be north or south at noon - depending on the date - and twice a year it will be overhead. Roger (talk) 21:15, 14 February 2011 (UTC)
agate spatula
[edit]Why is the instrument named agate spatula?? what is the reason 4 it?? — Preceding unsigned comment added by Suprithmurthy (talk • contribs) 04:31, 13 February 2011 (UTC)
- It's called an agate spatula because it is made of agate. Apparently these are used by dentists to mix cements, and a metal spatula would be bad because it could react with the cement. Looie496 (talk) 05:01, 13 February 2011 (UTC)
Name for an auction method
[edit]My friend described a kind of auction in game theory/economics to me, but I don't know what it's called, and it doesn't seem to be any of the ones described in Auction theory. Here's how it happens:
The seller sets a secret reserve price for the item. Each bidder puts a secret bid in an envelope. The envelopes are opened, and if any bid meets or exceeds the reserve price, the highest bidder wins, but that bidder pays only the reserve price, rather than their bid. If no bid meets the reserve, the item remains unsold.
What is this kind of auction called? —Keenan Pepper 05:42, 13 February 2011 (UTC)
- How could this auction work? After the auctioneer has all the bids, he would secretly sneak a peek in the envelopes of a few predicted highest bidders, and set the "reserve price" to just below the highest bid he sees. Alternatively, he'd tell a friend what the reserve price was, and that friend, confident that it was fairly low, could submit an absurdly high bid and clinch the item. Even an honest auction of this type leaves the seller in the peculiar position of accepting less money than the buyers are willing to bid. I have a hard time believing this is actually done - is this a real auction type or just a thought experiment for game theory? Wnt (talk) 06:24, 13 February 2011 (UTC)
- Right, it's not a practical method for real auctions. But what is it called? —Keenan Pepper 07:12, 13 February 2011 (UTC)
- If it's not practical, then why do you suppose anybody would bother to think of a name for it? –Henning Makholm (talk) 07:23, 13 February 2011 (UTC)
- Oh, don't be so naive. Lost of people spend their time thinking about unpractical things. Dauto (talk) 07:44, 13 February 2011 (UTC)
- Sure, but things don't usually get named until they've been thought about by several people (who know about the previous thoughts). This is the kind of auction idea that would get thought about once, rejected as impractical, and then not thought of again. --Tango (talk) 21:22, 13 February 2011 (UTC)
- It seems to be a model used by game theorists. There is more than one game theorist in this world and sometimes they like talking to each other. Dauto (talk) 23:35, 13 February 2011 (UTC)
- Sure, but things don't usually get named until they've been thought about by several people (who know about the previous thoughts). This is the kind of auction idea that would get thought about once, rejected as impractical, and then not thought of again. --Tango (talk) 21:22, 13 February 2011 (UTC)
- Oh, don't be so naive. Lost of people spend their time thinking about unpractical things. Dauto (talk) 07:44, 13 February 2011 (UTC)
Just to be sure...your friend isn't just getting themselves a bit muddled? What they describe sounds quite like a Vickrey auction - except that very last bit...that is to say in a `Vickrey auction' the winning person doesn't pay what they bid, they pay whatever the second highest bid was. Could it be that this is what they were describing? In the auction idea you describe I can't quite figure out what incentive people have to bid low assuming they want to win the item? ny156uk (talk) 09:14, 13 February 2011 (UTC)
- Yes, but if they bid a gazillion dollars they may end up having to pay 0.9 gazillion dollars (if that happens to be the secret reserve price) and that might be just a little too much to pay for the used thingamajig he is bidding on. Dauto (talk) 15:23, 13 February 2011 (UTC)
- Right, exactly. In fact I believe in this kind of auction the bidders have an incentive to bid their true value, which is why I think it's probably been discussed in a game theory context. —Keenan Pepper 17:48, 13 February 2011 (UTC)
- How does this method differ (in a material way) from simply setting a price, telling people what it is and letting someone buy it at that price if they so wish? --Tango (talk) 21:22, 13 February 2011 (UTC)
- Don't you think the knowledge of the set price will influence how high people are willing to bid? Dauto (talk) 23:51, 13 February 2011 (UTC)
I agree that this sounds like a second-price auction. As noted above, the equilibrium in this auction is for all players to bid their true valuations (assuming the standard economic utility framework). The more familiar first-price auction provides an incentive for the winning player to over-bid (the "winner's curse.").
As to the auction described, it seems it would be unworkable if more than one bidder valued the item greater than the reserve price. Each bidder will attempt to outbid the other, without constraint (since they only pay the reserve price no matter how high they bid). There is no Nash Equilibrium to this game since the strategy space is infinite and unbounded. It's a little like playing poker for a set number of dollars per hand and an infinite bankroll; the strategic possibilities would break down very quickly. —Preceding unsigned comment added by 12.186.80.1 (talk) 16:07, 15 February 2011 (UTC)
- It seems like nobody even READ what I wrote (except Dauto). The reserve price is SECRET. Each bidder ONLY BIDS ONCE. —Keenan Pepper 17:33, 18 February 2011 (UTC)
Smallpox Vaccine
[edit]What is the big needle used for when giving a smallpox vaccine?
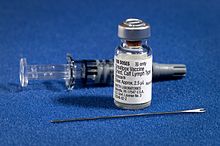

Also, why do people who have gotten the vaccine have a scar on their shoulder? Scar on Shoulder —Preceding unsigned comment added by 76.169.33.234 (talk) 07:53, 13 February 2011 (UTC)
- Sorry - I took the liberty to change your figures over to the standard thumbnail format, because they were too big and foul up display of the Refdesk.
I didn't know that by using a single bracket http link to the thumbnail you can get them to display,but the humdrum way is more practical. Wnt (talk) 07:59, 13 February 2011 (UTC)- Thanks! I couldn't figure it out, first it was too big, then it was just links. Looks much better now. —Preceding unsigned comment added by 76.169.33.234 (talk) 08:01, 13 February 2011 (UTC)
- You may be interested in Smallpox vaccine#Post-eradication vaccination Nil Einne (talk) 08:17, 13 February 2011 (UTC)
- The reason for the needle's shape and how its used is given in the article Bifurcated needle.--Aspro (talk) 11:23, 13 February 2011 (UTC)
- Thanks for the info. The article states that the established method was to do it in the left arm, was there any particular reason for this? Why not do it someplace else where the scar that is left is not visible? Is the permanent scar that is left because of the vaccine or because of the needle? —Preceding unsigned comment added by 76.169.33.234 (talk) 11:53, 13 February 2011 (UTC)
- The scar results because the vaccine is a live virus, which multiplies at the site of introduction (in the cells around the skin puncture). One's immune system then kicks in and confers immunity which prevents the virus spreading and causing more 'pox marks.' This local infection is what causes a scab to form, which eventual drops off. The needle just needs to puncture the skin enough to see a little blood. This is because unbroken skin is a very good barrier and may prevent the virus from successfully invading the live skin cells below. The tiny puncher marks on their own, would otherwise heal without a trace. So it is the infection that leaves the scare.
- People who have had small pox, very often carry pox marks for the rest of their lives.
- The inoculation is performed on the opposite side to the dominant hand because as it is a live virus and a real irritating infection, the site is commonly tender and sore -and can itch like mad. In a few people, it can ache so much that they like to have the arm in a sling. Hence the preference for the arm on the opposite side. It is for this reason too that it is better on the arm than the leg. Also, there is the practical aspect that in an inoculation drive, it is more convenient to have people (maybe a thousand or more in a day) form a line with their sleeves rolled up. One doesn't have to lean too far down to reach each arm. You never find medical students picking grapes on their vaccation – too much bending!--Aspro (talk) 15:32, 13 February 2011 (UTC)
- If the smallpox vaccine were still given today routinely like it was in the past, do doctors now have something that they can give to the patients to minimize the scars? --173.49.14.8 (talk) 04:30, 14 February 2011 (UTC)
- In the past the scars were not considered an issue because almost everybody had one, so I would image they would be dismissed in the same way today. The only treatment I can think of, that may reduce scaring, would be to stop the blister from drying out and forming a hard scab. This could be done by applying silicone scar sheets and then continuing for some time after the dead skin sloughs off, with the topical application of a suitable skin gel. Other treatments such as ex-foliation of the skin etc., to encourage it to regrow smooth is I think – vanity going too far.--Aspro (talk) 11:37, 14 February 2011 (UTC)
- If the smallpox vaccine were still given today routinely like it was in the past, do doctors now have something that they can give to the patients to minimize the scars? --173.49.14.8 (talk) 04:30, 14 February 2011 (UTC)
- Note that the smallpox vaccine isn't the only vaccine which produces a scar. The Bacillus Calmette-Guérin is also know for the scarring although that is usually applied to the upper arm and with a more normal hypodermic needle (at least it was in Malaysia) AFAIK. The BCG uses attenuated live bacteria however a live vaccine obviously doesn't guarantee prominent/significant scarring as it depends on specific vaccine and the bodies response thereof, for example I don't believe the MMR vaccine is notable for any particular scarring. Nil Einne (talk) 07:24, 14 February 2011 (UTC)
Vasectomy: Potential post-operative hormonal changes
[edit]The standard article on vasectomy states that after the procedure, "Sperm are still produced by the testicles, but they are broken down and absorbed by the body." Several external articles (which may or may not be objective) put the "average time" for spermatozoa to be reabsorbed into the body after production at around 40 days (but their cited source is obscure). Many papers are available on vasectomy itself, post-operative complications and chronic pain, but I am unable to determine whether any studies have been conducted on post-operative hormonal changes. My question pertains to whether male hormonal levels (specifically those of androgens such as androstenedione and testosterone) are likely to increase measurably after vasectomy, given that sperm cells and their constituent elements are no longer being expelled from the body, but broken down and re-used. Any study or article that shows measurable change (of even the smallest degree) might be relevant; failing that, even anecdotal evidence might be of use, provided that its evidential value is reinforced by multiple instances. Consulting urological surgeons has not yielded results, since their speciality might be said to lie in the performance of the procedure, not its effect on the delicate balances of blood chemistry.
Malusmoriendumest (talk) 08:05, 13 February 2011 (UTC)
- All I can give is my opinion. I would say no, it shouldn't have any affect on hormone levels, since breaking down of cells, such as sperm, would involve them dying, being disassembled into base components, then reassembled into other things or excreted. StuRat (talk) 04:02, 14 February 2011 (UTC)
Temperature and wavelengths
[edit]Is it possible to contain all wavelengths/frequencies at a specific temperature? For example does Sun emit frequencies in the range of 0 to ∞ Hz, and does our body do the same thing?--Email4mobile (talk) 08:33, 13 February 2011 (UTC)
- I think I couldn't find a clear answer in that article. Let me give a direct example: Is heating iron to 1000 °C associated with Gamma rays emissions or Radio frequency waves?--Email4mobile (talk) 10:18, 13 February 2011 (UTC)
- I was reluctant to say anything more because I don't want to risk saying anything false about extremely high energy photons. Anywaw, as far as black body radiation applies, any body at nonzero temperature emits radiation at all wavelengths, so the answer to your original question is yes (you personally emit some extremely small amount of gamma rays). Nevertheles, most of the radiated energy goes into radiation at a certain frequency range that is a function of the body's temperature. So 1000°C emits mostly visible light and infrared, but it also emits some negligibly small amount of radio waves and gamma rays. 83.134.173.228 (talk) 11:07, 13 February 2011 (UTC)
- The amount of radio waves is not negligible. Dauto (talk) 15:06, 13 February 2011 (UTC)
- The article on Planck's law shows the rather slewed bell curve of the spectral output verses temperature. It is the peak of the curve that corresponds to a specific temperature.--Aspro (talk) 11:37, 13 February 2011 (UTC)
- I was reluctant to say anything more because I don't want to risk saying anything false about extremely high energy photons. Anywaw, as far as black body radiation applies, any body at nonzero temperature emits radiation at all wavelengths, so the answer to your original question is yes (you personally emit some extremely small amount of gamma rays). Nevertheles, most of the radiated energy goes into radiation at a certain frequency range that is a function of the body's temperature. So 1000°C emits mostly visible light and infrared, but it also emits some negligibly small amount of radio waves and gamma rays. 83.134.173.228 (talk) 11:07, 13 February 2011 (UTC)
- I haven't worked out the numbers for when this would happen, but if you have a cold enough object and you are interested in a short enough wavelength then, while technically some radiation at that wavelength will be emitted by that object, it may work out as less than one photon during the entire age of the universe. That can be interpretted as essentially not emitting anything at that wavelenght. --Tango (talk) 21:31, 13 February 2011 (UTC)
- I was interested in this question, and my back-of-envelope calculation suggests that for normal-sized black-body objects at around room temperature, this cutoff point, beyond which a total (over all wavelengths) of less than one photon is emitted during the entire age of the universe so far, lies somewhere in the visible spectrum. (Don't rely on this answer though ... I could have done something drastically wrong!) 86.160.216.197 (talk) 21:40, 14 February 2011 (UTC)
Thanks for the answers. Indeed I was thinking something similar and wanted to share the question before I submit the answer in Arabic for someone asked in Google Answers (Google opened Arabic service less than two years ago). I can understand that even if there were gamma or RF waves, they would be negligible in the hole spectrum at temperature ranges less than several thousands °C.--Email4mobile (talk) 22:45, 15 February 2011 (UTC)
which software
[edit]please any experienced person help me .. which software i should i use to model a disintegration of nuclear fuel rod(fission process).please give any other useful regarding its modelling —Preceding unsigned comment added by 59.93.130.74 (talk) 09:33, 13 February 2011 (UTC)
- There are different Nuclear fuel cycles. This article may help you focus in on the one that suits your application best. Then you can start looking for the software that models that cycle. Thorium is thought to be the next big thing in reactor design. --Aspro (talk) 11:42, 13 February 2011 (UTC)
- Perhaps User:Rocketshiporion can help? Their interest seemed to be in the nuclear weapon side but they may have picked up something in their research that may be of use Nil Einne (talk) 16:08, 14 February 2011 (UTC)
- If you mean the physical disintegration of the nuclear fuel rod (which AFAIK shouldn't happen in a properly designed reactor), see Field Precision's GamBet Monte Carlo Suite. If OTOH you mean modeling the operation of a nuclear reactor, you probably need something like this, but unfortunately it is not commercially available. Rocketshiporion♫ 05:12, 17 February 2011 (UTC)
physics
[edit]what is horizon of a time machine? — Preceding unsigned comment added by Sanandnps (talk • contribs) 14:38, 13 February 2011 (UTC)
- You can't title your question "Physics" and then ask about time machines. Since time machines only exist in fiction, the entertainment forum might be a better place for your question. Dauto (talk) 15:46, 13 February 2011 (UTC)
- Why answer so rudely? 1) Time travel is a very real topic in physics, and the time travel article has dozens of references from peer-reviewed physics journal articles, 2) if you don't have an answer, why post? — Sam 67.186.134.236 (talk) 18:17, 13 February 2011 (UTC)
- I didn't mean to be rude., I think my answer is the best answer to the question. Dauto (talk) 20:05, 13 February 2011 (UTC)
- That depends. Is the hypothesized time machine traveling forward or backward in time? If traveling forward in time (faster than normal), one way to do that would be to hang out just outside the event horizon of a black hole, as mentioned in Time travel#Time travel to the future in physics. Traveling back in time may or may not be possible, depending on whether closed timelike curves actually exist. The boundary of a set of closed timelike curves is a Cauchy horizon, which is touched on lightly in Time travel#Other approaches based on general relativity. Red Act (talk) 16:21, 13 February 2011 (UTC)
- By the way, even if travel along a closed timelike curve is possible (and there are some very strong constraints on that possibility, such as Hawking's constraint prohibiting a compactly generated Cauchy horizon in a region where the weak energy condition is satisfied), the nature of doing that would be very different from the type of time travel that's pretty much always depicted in fiction, in which the time machine or time traveler has a discontinuous world line. A discontinuous jump in a world line like that is just fiction. Red Act (talk) 17:38, 13 February 2011 (UTC)
- Dang, I just wish for the days when all you had to do was push this little button and you appeared in a futuristic city filled with Eloi. Crimsonraptor | (Contact me) Dumpster dive if you must 18:46, 13 February 2011 (UTC)
- By the way, even if travel along a closed timelike curve is possible (and there are some very strong constraints on that possibility, such as Hawking's constraint prohibiting a compactly generated Cauchy horizon in a region where the weak energy condition is satisfied), the nature of doing that would be very different from the type of time travel that's pretty much always depicted in fiction, in which the time machine or time traveler has a discontinuous world line. A discontinuous jump in a world line like that is just fiction. Red Act (talk) 17:38, 13 February 2011 (UTC)
- Its horizon seems to be only a few seconds - yours, a dissatisfied customer.
- Dear Sir: I am writing to complain about the Mark I time machine that you sold me ... Gandalf61 (talk) 13:36, 14 February 2011 (UTC)
Pig feed
[edit]What are domesticated pigs at pig farms fed? (historical info would be interesting as well)Mintyf (talk) 15:41, 13 February 2011 (UTC)
- It's unsourced and not terribly detailed, but we do have some information in the second paragraph of Intensive_pig_farming#Intensive_piggeries and at Sty#Slopping_the_Hogs. --Allen (talk) 18:24, 13 February 2011 (UTC)
- They're fed pelletized grain. --Sean 15:38, 14 February 2011 (UTC)
- For historical information, Toilets_in_Japan#History states that:In Okinawa, the toilet was often attached to the pig pen, and the pigs were fed with the human waste product. This practice was banned as unhygienic after World War II by the American authorities....though I don't believe that practice was widespread.Smallman12q (talk) 19:28, 14 February 2011 (UTC)
What is this plant?
[edit]http://img.skitch.com/20110213-mykikb758gb8g8si9q5kh865gi.jpg
Hi all,
Does anyone know what this plant is? We were thinking it was a Wandering Jew, but now I don't think it looks that much like one. Thanks so much! — Sam 67.186.134.236 (talk) 18:07, 13 February 2011 (UTC)
- I know it as a Wandering Jew too, but I think it's one of the Tradescantia family. --TammyMoet (talk) 18:28, 13 February 2011 (UTC)
- Maybe specifically Tradescantia spathacea—cf. this Flickr image. Deor (talk) 18:34, 13 February 2011 (UTC)
- Thanks! — Sam 67.186.134.236 (talk) 21:15, 13 February 2011 (UTC)
- Maybe specifically Tradescantia spathacea—cf. this Flickr image. Deor (talk) 18:34, 13 February 2011 (UTC)
Low-density solids/liquids?
[edit]Are there any solids or liquids that are less dense than air? --75.15.161.185 (talk) 21:01, 13 February 2011 (UTC)
- Our article Aerogel has some information on this topic:
- "The world's lowest-density solid is a silica nanofoam at 1 mg/cm3, which is the evacuated version of the record-aerogel of 1.9 mg/cm3. The density of air is 1.2 mg/cm3."
- So it seems there are solids lighter than air, but they're highly porous materials.
- Ben (talk) 21:09, 13 February 2011 (UTC)
- So why don't they float off into space? --75.15.161.185 (talk) 21:16, 13 February 2011 (UTC)
- I think "evacuated version" means what you get when you put it in a vacuum. In air, it has a density higher than air so doesn't float away. In a vacuum, there is nothing for it to float in. --Tango (talk) 21:34, 13 February 2011 (UTC)
- so then in other words, because the structure of an aerogel is always "thin strands of open-cell structure of something, with air inbetween," the answer is no? 65.29.47.55 (talk) 22:58, 13 February 2011 (UTC)
- I think "evacuated version" means what you get when you put it in a vacuum. In air, it has a density higher than air so doesn't float away. In a vacuum, there is nothing for it to float in. --Tango (talk) 21:34, 13 February 2011 (UTC)
- So why don't they float off into space? --75.15.161.185 (talk) 21:16, 13 February 2011 (UTC)
So are there any solids or liquids that are less dense than air when in the atmosphere (i.e. not in a vacuum)? --75.15.161.185 (talk) 23:01, 13 February 2011 (UTC)
- I think that you should relax your standards to allow for any gas, and it will be hard to find such a case anyway. If you do this, then a gas like sulfur hexafluoride (or for those willing to put up with nasty nasty HF, tungsten hexafluoride) can be up to ten times denser.
- Now an aerogel is not "truly" solid throughout; it's a sort of mass of fibers. If you allow something not solid throughout, you might as well allow a boat. Which brings us to [1], which is about as close as I think you're going to get to what you want. Wnt (talk) 00:01, 14 February 2011 (UTC)
- Instead of going with another gas, you could go with more pressure. This would compress air more than most liquids or solids, thus making them more likely to float. StuRat (talk) 03:55, 14 February 2011 (UTC)
- I think the question is nonsensical, imagine asking the opposite: is there a gas more dense then solid? Obviously not; if it was more dense, then it would BE solid. Vespine (talk) 04:52, 14 February 2011 (UTC)
- I don't think that follows: for example liquid Mercury is much more dense than solid Sodium at room temperature. AndrewWTaylor (talk) 09:25, 14 February 2011 (UTC)
- The question and most of the discussion is about "solids and liquids" vs gases, not "solids vs liquids". Solids and liquids generally do have similar densities (molecules just less organized and a little less tightly attached to each other in liquids), whereas gases are generally orders of magnitude less dense (molecules highly spaced) at normal pressures and temperatures. DMacks (talk) 16:44, 14 February 2011 (UTC)
- I don't think that follows: for example liquid Mercury is much more dense than solid Sodium at room temperature. AndrewWTaylor (talk) 09:25, 14 February 2011 (UTC)
- I think the question is nonsensical, imagine asking the opposite: is there a gas more dense then solid? Obviously not; if it was more dense, then it would BE solid. Vespine (talk) 04:52, 14 February 2011 (UTC)
- One thing worth noting is that the definition of a "solid" is a wee bit unclear. The old rule-of-thumb "conforms to the shape of its container" (like water becomes a cylinder when poured into a glass) becomes a bit weird for gels, infusions, and glasses (silica glass has a barely-measurable viscosity). Molecularly, one might call a solid as being a stable crystal lattice, of which there are many configurations like the sorts in the phase diagram of water/ice, while polymers and glasses would be amorphous solids (or, in physics, soft condensed matter).
- The point is that aerogel is a solid as most would think of it, while a highly porous object, like the very light pumice rock, is cheating a bit (especially if we filled the holes with helium and sent it up like a zeppelin. But if you want a nice crystal structure, then here's a fun exercise: check out the [formula for theoretical density of a crystal] and compare it to the ideal gas law to see if you can get your very low-density configuration of some light atom or molecule - the point of the ideal gas law is it sets an upper limit on your solid's volume. SamuelRiv (talk) 23:39, 14 February 2011 (UTC)
- For a theoretical solid you could try solid dipositronium, unfortunately it decays too fast to be realised, but it would be lighter than air and solid, but at cryogenic temperatures. Graeme Bartlett (talk) 23:52, 14 February 2011 (UTC)
