Wikipedia:Reference desk/Archives/Science/2009 August 24
| Science desk | ||
|---|---|---|
| < August 23 | << Jul | August | Sep >> | August 25 > |
| Welcome to the Wikipedia Science Reference Desk Archives |
|---|
| The page you are currently viewing is an archive page. While you can leave answers for any questions shown below, please ask new questions on one of the current reference desk pages. |
August 24
[edit]Digging yourself out of a hole
[edit]If I was in a hole that was say, 10ft deep and 4ft wide and only had a shovel could I dig myself out? I've been thinking about this for ages and everyone I ask has a different opinion! Has anyone ever done it? Thanks Smartse (talk) 03:09, 24 August 2009 (UTC)
- You could dig away at the walls to continually add dirt to the floor, thereby making the pit more shallow as you dig, until you could climb out. DRosenbach (Talk | Contribs) 03:16, 24 August 2009 (UTC)
- I can't think of why you "couldn't" dig your self out? The hole is 4 foot wide, that's pretty narrow, i imagine it would be difficult to manipulate a shovel which is probably at least 4 foot long, but you don't need to have the shovel horizontal. As the above says, scrape away at the sides to fill the hole up with dirt and eventually just climb out, might not be "easy" but i don't see anything impossible about it. It's not like picking yourself up by the shoe laces. Being 10 foot deep i suppose you should also worry about caving the hole in around you if you dig away at one side too much, which would be bad. Vespine (talk) 04:24, 24 August 2009 (UTC)
- Caving in would be the problem, all right. If you could reach the top of the hole with the shovel there would be no problem: widen the hole at the top so that earth falls to the bottom, and then stand on this earth. But in a 10-foot hole you can't reach the top, so the best you can do is excavate the sides to get earth to stand on. But then this creates an overhang, which may be unstable. It depends very much on the characteristics of the earth that the hole is in, but since the problem is hypothetical in the first place, we don't know about that. You might be able to excavate the sides of the hole enough without collapsing the ground above you, or you might not.
- It's also possible with a hole only 10 feet deep that even if you did cause a cave-in, it still might not keep you from getting out, because the amount of earth that spills into the hole might not be enough to hurt you. If you can extract yourself (and the shovel) from the collapsed earth and stand on top of it, you now have a shallower hole -- repeat the process and you're out.
- --Anonymous, 05:01 UTC, August 24, 2009.
- "But in a 10-foot hole you can't reach the top..." -- If you're 6 feet tall and got a 4-foot-long shovel, then yes you can. 98.234.126.251 (talk) 05:12, 24 August 2009 (UTC)
- Hmm, good point. I wasn't thinking of the shovel as being usable that far overhead, but even if you can't quite reach the top, you can get near enough that a cave-in of the earth above where you're digging would not release enough earth to bury you. So unless you or the shovel are very short, the answer is yes, you should be able to dig out by working on the walls as high as you can reach. --Anon, 16:18 UTC, August 24, 2009.
- A 4ft shovel seems pretty long and remember that your shoulder is ~1ft below your head. Smartse (talk) 19:52, 24 August 2009 (UTC)
- Yes, "your shoulder is ~1ft below your head", but why would you have to hold the shovel from shoulder height (i.e., not exteding arms above level)? I can reach my hands over a foot above the top of my head. DMacks (talk) 20:00, 24 August 2009 (UTC)
- A 4ft shovel seems pretty long and remember that your shoulder is ~1ft below your head. Smartse (talk) 19:52, 24 August 2009 (UTC)
- Hmm, good point. I wasn't thinking of the shovel as being usable that far overhead, but even if you can't quite reach the top, you can get near enough that a cave-in of the earth above where you're digging would not release enough earth to bury you. So unless you or the shovel are very short, the answer is yes, you should be able to dig out by working on the walls as high as you can reach. --Anon, 16:18 UTC, August 24, 2009.
- "But in a 10-foot hole you can't reach the top..." -- If you're 6 feet tall and got a 4-foot-long shovel, then yes you can. 98.234.126.251 (talk) 05:12, 24 August 2009 (UTC)
- (After e/c)x2
- IF you were careful, I don't see why not. Your goal would be to 1) Remove dirt from the sides of the hole and place it under your feet at the bottom of the hole. and 2) Make the sides of the hole less steep. With a full length shovel you could reach the rim of the hole, so you could widen the hole gradually all the way from the top. (Don't try to "tunnel"; It'll cave-in.) Eventually you'd wind up with a nice cone-shaped hole about half as deep as the original hole. At that point you could just walk out. APL (talk) 05:03, 24 August 2009 (UTC)
- You would want strategic digging for controlled cave-ins, to create the sloped, cone-like walls that APL is mentioning. This would require some careful planning and estimation. A catastropic cave-in could easily release enough material to smother you. By studying the way the wall reacts to your digging, I think you could carefully arrange for controlled slides. You'd get pretty dirty, though - even if not you weren't buried, debris from these cave-ins would definitely get on you. Here's a book, partially available online: Rock slope engineering. Chapter 12 has some theory overview on stability considerations for different shaped rock or sediment walls. For a more basic introduction, you can read Angle of repose - this is the maximum stable angle that a particular mix of dirt/rock prefers to take. You want to work the wall of your hole towards that angle - it's currently in an unstable state (assuming it's got "vertical" walls). We also have an article on geotechnical engineering, which has some content related to sediment stability. Nimur (talk) 05:39, 24 August 2009 (UTC)
- Seems to me there's a middle position where you'd be standing in hole with a diamond-shaped or kite-shaped cross-section, maybe ten feet across at its widest point, with a large amount of earth about to fall on you. (Also, if instead of instead of aiming at building a slope, you tried the simpler method of removing earth from the sides and flattening it out under your feet, and assuming no cave-ins, there's a Zeno's arrow or limit type of situation where although you keep raising yourself higher the rate of ascent slows down due to the widening of the hole, and you never mathematically reach the top.) 213.122.42.252 (talk) 00:03, 25 August 2009 (UTC)
- You don't have to "mathematically" reach the top -- the point of the whole exercise is to get yourself to where you can climb out of the hole with no further assistance. 98.234.126.251 (talk) 00:15, 25 August 2009 (UTC)
- Yes, because people have a height. Just as well, really. Tell you what though, if you were a small person with a short shovel then getting out of the hole might take an awkwardly long time, what with progress slowing down as the hole gets wider. 213.122.42.252 (talk) 00:23, 25 August 2009 (UTC)
- You don't have to "mathematically" reach the top -- the point of the whole exercise is to get yourself to where you can climb out of the hole with no further assistance. 98.234.126.251 (talk) 00:15, 25 August 2009 (UTC)
- Seems to me there's a middle position where you'd be standing in hole with a diamond-shaped or kite-shaped cross-section, maybe ten feet across at its widest point, with a large amount of earth about to fall on you. (Also, if instead of instead of aiming at building a slope, you tried the simpler method of removing earth from the sides and flattening it out under your feet, and assuming no cave-ins, there's a Zeno's arrow or limit type of situation where although you keep raising yourself higher the rate of ascent slows down due to the widening of the hole, and you never mathematically reach the top.) 213.122.42.252 (talk) 00:03, 25 August 2009 (UTC)
- Why dig? With a four-foot diameter hole you can walk out. Put your back to one side of the hole, lean forward and place your hands on the other side of the hole. Now walk up. 152.16.59.102 (talk) 05:53, 24 August 2009 (UTC)
- Climbing_technique#Chimneying requires fairly strong material--gotta be able to hold you up, not just its own weight against a cave-in or other slide. On the other hand, the lateral force as one presses against the dirt. DMacks (talk) 06:15, 24 August 2009 (UTC)
- With the exception of extremely sandy soil or swampland, the chimneying technique should work just about anywhere. I've dug enough foxholes in my life to be certain that the tightly-packed dirt just under the surface would support the average person's weight. It is nothing like the loose topsoil at the surface. 152.16.59.102 (talk) 06:46, 24 August 2009 (UTC)
- Climbing_technique#Chimneying requires fairly strong material--gotta be able to hold you up, not just its own weight against a cave-in or other slide. On the other hand, the lateral force as one presses against the dirt. DMacks (talk) 06:15, 24 August 2009 (UTC)
- It's also possible to dig even deeper on half of the hole and use that material to build steps up on the other half of the hole. That might buy you 2 to 3 feet that would go a long way towards reaching the top and start widening it to fill the bottom. Dauto (talk) 06:04, 24 August 2009 (UTC)
- If it's a good sturdy shovel you might be able to hold it overhead (at, say, 7 feet from the bottom), wedge it between the walls, and then climb onto it and out. But that would be riskier than digging, so use it as a last resort. --Sean 13:37, 24 August 2009 (UTC)
Thanks for the answers! Unfortunately I have kind of lost an argument but at least I know now. It seems as though it definitely does depend on the size hole then as if it where deeper than 10ft you wouldn't be able to reach the top. Can anyone find information on whether anyone has actually managed to do it or will I have to test the theory myself one day? Smartse (talk) 19:52, 24 August 2009 (UTC)
- I would not advise you go try this in a personal experiment. Dry soil generally has a density of about 100lb/ft^3. a small slide could easily have over a ton of soil and you could get buried alive. Googlemeister (talk) 20:39, 24 August 2009 (UTC)
- Yes, people digging even shallow ditches sometimes get buried alive. If alone they're probably done for. Even with helpers, digging them up (at least enough so they can breathe) before they suffocate is a challenge. StuRat (talk) 21:30, 24 August 2009 (UTC)
- I'm not stupid! Don't worry I'm nowhere near a hole at the moment, I just wondered if anyone has actually done it. The theory sounds very convincing but if no one has done it should I really believe the answers? Are you sure about that density too - that is pretty heavy soil where you live. Smartse (talk) 22:13, 24 August 2009 (UTC)
- Water weighs roughly 64lb/ft^3 and the soil article reports that it varies between one and two times the density of water, so a density of 1.5gm/cm^3 doesn't sounds particularly heavy. -- Thinking of England (talk) 11:00, 25 August 2009 (UTC)
Responders seem to assume that the OP finds themself in a hole extending downwards in earth that can be shovelled aside but there are other possibilities. If thinking about the situation has been obsessive then it seems to be more a problem in psychology than earth moving. Being buried alive is said to be one of the most widespread of human fears. Cuddlyable3 (talk) 00:43, 25 August 2009 (UTC)
- Alternatively, if the hole has a diameter some amount less than the length of your shovel and the hole is less than two body lengths high, you can raise the shovel up to shoulder height, dig the spade end horizontally into the dirt and create a nearly horizontal bar across the hole. Climb up onto that, and climb out. Mac Davis (talk) 14:48, 25 August 2009 (UTC)
extraction of MGO/CACO3 from DOLOMITE/CALCITE
[edit]is it possible to extract MGO from DOLOMITE ,CACO3 from CALCITE on a comercial basis ? GRIPTOR (talk) 08:10, 24 August 2009 (UTC)
FYI, calcite is CaCO3. As for extracting MgO from dolomite: yeah, you could do it easy enough; the only hard part would be separating it from the calcium. You could do it (for instance) by dissolving the dolomite in acid and selectively precipitating either the calcium or the magnesium to separate the two. 98.234.126.251 (talk) 09:07, 24 August 2009 (UTC)
- You could calcine the dolomite to turn it to magnesium oxide and calcium oxide. On solution with water, the calcium oxide is more soluble. Alternatively if you dissolve in hydrochloric acid to form magnesium chloride, you can precipitate the magnesium hydroxide by treating it with lime. By heating the magnesium hydroxide you can get back the Magnesium oxide. However this last method would not be economical due to the cost of the acid. You can get magnesium chloride from sea water more cheaply. you may wish to read this: [1]. There could still be impurities such as iron and nickel oxides. Graeme Bartlett (talk) 12:39, 25 August 2009 (UTC)
Calculating cumulative U-values
[edit]I have two units of insulation; one being 100mm with a U-value of 0.494 W/m2K and another, on top, 200mm thick with a value of 0.140 W/m2K. I'm not sure how to calculate the cumulative U-value. I assumed I could take the reciprocal of both, add them together (to get 9.16) then take the reciprocal of this value to obtain a cumulative U-value. Is this correct? —Preceding unsigned comment added by 157.203.42.175 (talk) 09:10, 24 August 2009 (UTC)
- That sounds to be the right method. If you used the R values you would just add. Graeme Bartlett (talk) 09:27, 24 August 2009 (UTC)
Bouncing X-rays
[edit]It is my understanding that x-rays either penetrate or are absorbed, but do not bounce. Is that an oversimplification, or perhaps not even true -- the article makes no mention as far as I can see. My point is that there are these dental assistants who insist on closing the door to the operatory when exposing patients to x-rays, even when the cone is clearly pointing in a direction such that the doorway is in no way in the beam from the machine. DRosenbach (Talk | Contribs) 13:53, 24 August 2009 (UTC)
- To some extend they do bounce back. Otherwise [| X-Ray diffraction] would not be possible. The only part about which one could argue would be how harmful those backscattered rays would be. --91.6.61.154 (talk) 14:06, 24 August 2009 (UTC)
- (edit conflict).Mostly they do not bounce - however they can be 'reflected' - using diffraction (an array of silicon crystals is sometimes used to focus x-rays) - that is irrelevant here. I would expect that the assistants are just being over cautious - closing the door may partially prevent some body innadvertantly wandering in when the x-rays are being used, or perhaps to give the patient a little 'privacy' when they are undergoing what is technically an invasive procedure.
- On absorbtion of x-rays secondary emission often occurs - a less energetic ray (or particle) can be emitted randomly from the thing it absorbed - this is scattering.
- It's mostly likely psychology rather than science.83.100.250.79 (talk) 14:08, 24 August 2009 (UTC)
- X-rays can also be reflected by metallic surfaces if they are incident at a very shallow grazing angle. This type of reflection is used in X-ray telescopes. See this page for some diagrams. -- Coneslayer (talk) 14:20, 24 August 2009 (UTC)
- Thanx for your answers -- I did forget to mention backscatter potential in the question -- but I was sort of hoping for someone to confirm that it is psychology rather than probably psychology before I go around telling everyone that they're being silly. In principle, I'm against the propagation of ignorance and it really gets me when the assistants threaten to "tell on the students and the residents" when they fail to close the door, thus exposing everyone in the hall and causing undue cases of extra cancer. Can I think of x-rays as billiard balls and the operatory as a pool table, and make sure that as long as the x-rays won't bounce to the door in one bounce, I'll be OK. I mean, certainly after hitting 2 or more walls, the intensity of the rays has dissipated to below even measurable magnitudes -- and that would be even when the wall is metal. DRosenbach (Talk | Contribs) 14:21, 24 August 2009 (UTC)
- Your x-ray lab will have safe operating rules. Do these specify whether the door must be closed? AlmostReadytoFly (talk) 14:36, 24 August 2009 (UTC)
- That's just it -- school regulations are based on ignorance, or at least that's what I'm trying to establish. I'm interested in science, not bureaucracy. DRosenbach (Talk | Contribs) 15:10, 24 August 2009 (UTC)
- I don't think it's possible to say beyond a doubt there's no risk since as has been mentioned, there's likely some risk and a large number of variables. Assessing that risk is probably the job of an actuary who may be able to make a resonable assessment, particularly if you limit the variables (e.g. for a specific location) but that's likely a costly process. It's far easier to presume the risk is non negible and take precautions which don't have much of a negative effect then run the risk that something does go wrong and the legal and financial consequences thereof (particularly in the US). This isn't so much bureaucracy as following the cheapest and easiest pathway and there are parallels in many areas of OSH as well as in other areas of life, e.g. mobile phone use in aeroplanes. There are likely also some other concerns that haven't been mentioned, e.g. while I presume there are warning lights when the X-ray is in use, a closed door adds an extra precaution against someone walking in by accident or another e.g. it may be comforting to the people being X-rayed. BTW the psychology element is also not irrelevant since even ifan actuary does decide the risk is less then 1 in 1 trillion, people are notoriously bad at understanding risks and many may still be worried so those writing the rules would likely think it wise give those who worry piece of mind given the small cost to everyone involved. In any case, while you're obviously welcome to challenge any inaccurate claims it seems to me as long as the rules are there, those who agree to follow the rules (and I'm sure anyone working or studying there must have to agree) should follow them, regardless of the reasons for following them. If you can come up with conclusive evidence the risk is too low to be of concern, you're far better off taking that to management and convincing them to change the rules rather then ignoring the rules and expecting everyone else to not care. Nil Einne (talk) 15:43, 24 August 2009 (UTC)
- There may also be regulatory issues that the place needs to comply with. APL (talk) 15:53, 24 August 2009 (UTC)
- Doors do not stop Xrays very well. Doors however stop people and closing doors is a way of stopping random innocents walking through them and into the path of the beam. Random innocents includes people who cannot read, who are walking backwards pulling a trolley etc etc. Unless the door is lead I think the reason for shutting the door is most likely to be people. As for the Xrays, at the point of generation they are probably going in all directions (from a high energy or radioactive source) so there will be a few heading for the door. --BozMo talk 16:05, 24 August 2009 (UTC)
- Just a note, BozMo: there is nothing magical about lead, as though this was x-ray vision. Aluminum attenuates x-ray beams, as does concrete. Lead just does attenuates very well even at minimal thicknesses, and so was used a lot early on. I'm in NJ, and I know the state just modified regulations for dental offices such that lead lines walls are no longer necessary for newly fabricated offices -- double sheet rock is now sufficient (I believe up to 7 feet or so). DRosenbach (Talk | Contribs) 18:39, 24 August 2009 (UTC)
- Doors do not stop Xrays very well. Doors however stop people and closing doors is a way of stopping random innocents walking through them and into the path of the beam. Random innocents includes people who cannot read, who are walking backwards pulling a trolley etc etc. Unless the door is lead I think the reason for shutting the door is most likely to be people. As for the Xrays, at the point of generation they are probably going in all directions (from a high energy or radioactive source) so there will be a few heading for the door. --BozMo talk 16:05, 24 August 2009 (UTC)
- There may also be regulatory issues that the place needs to comply with. APL (talk) 15:53, 24 August 2009 (UTC)
- I don't think it's possible to say beyond a doubt there's no risk since as has been mentioned, there's likely some risk and a large number of variables. Assessing that risk is probably the job of an actuary who may be able to make a resonable assessment, particularly if you limit the variables (e.g. for a specific location) but that's likely a costly process. It's far easier to presume the risk is non negible and take precautions which don't have much of a negative effect then run the risk that something does go wrong and the legal and financial consequences thereof (particularly in the US). This isn't so much bureaucracy as following the cheapest and easiest pathway and there are parallels in many areas of OSH as well as in other areas of life, e.g. mobile phone use in aeroplanes. There are likely also some other concerns that haven't been mentioned, e.g. while I presume there are warning lights when the X-ray is in use, a closed door adds an extra precaution against someone walking in by accident or another e.g. it may be comforting to the people being X-rayed. BTW the psychology element is also not irrelevant since even ifan actuary does decide the risk is less then 1 in 1 trillion, people are notoriously bad at understanding risks and many may still be worried so those writing the rules would likely think it wise give those who worry piece of mind given the small cost to everyone involved. In any case, while you're obviously welcome to challenge any inaccurate claims it seems to me as long as the rules are there, those who agree to follow the rules (and I'm sure anyone working or studying there must have to agree) should follow them, regardless of the reasons for following them. If you can come up with conclusive evidence the risk is too low to be of concern, you're far better off taking that to management and convincing them to change the rules rather then ignoring the rules and expecting everyone else to not care. Nil Einne (talk) 15:43, 24 August 2009 (UTC)
- That's just it -- school regulations are based on ignorance, or at least that's what I'm trying to establish. I'm interested in science, not bureaucracy. DRosenbach (Talk | Contribs) 15:10, 24 August 2009 (UTC)
- Your x-ray lab will have safe operating rules. Do these specify whether the door must be closed? AlmostReadytoFly (talk) 14:36, 24 August 2009 (UTC)
- Thanx for your answers -- I did forget to mention backscatter potential in the question -- but I was sort of hoping for someone to confirm that it is psychology rather than probably psychology before I go around telling everyone that they're being silly. In principle, I'm against the propagation of ignorance and it really gets me when the assistants threaten to "tell on the students and the residents" when they fail to close the door, thus exposing everyone in the hall and causing undue cases of extra cancer. Can I think of x-rays as billiard balls and the operatory as a pool table, and make sure that as long as the x-rays won't bounce to the door in one bounce, I'll be OK. I mean, certainly after hitting 2 or more walls, the intensity of the rays has dissipated to below even measurable magnitudes -- and that would be even when the wall is metal. DRosenbach (Talk | Contribs) 14:21, 24 August 2009 (UTC)
- Yes, x-rays can (and do) bounce. Generally the scattered intensity will be very low relatively to the strength of the primary beam, but cumulative exposure may be significant. A number of processes can contribute to x-ray scattering, but at the moderate energies used for medical x-rays I believe that Compton scattering] is the predominant process. (To be sure, check a copy of Eric Hall's Radiobiology for the Radiologist or similar.) Except under the very specific conditions described above (very shallow grazing incidence, or Bragg reflection from a crystalline material) it is unlikely that 'specular', highly-peaked reflections will be the major contributor to off-beam exposure. In other words, the 'bouncing billiard ball' model isn't going to work for you unless you mean to assume that the balls can bounce at any arbitrary angle off of any surface. (Even then, there will be a very small additional amount of scatter simply during passage through open air.)
- Oftentimes, radiation protection practices follow the ALARA ('as low as reasonably achievable') principle. As it is virtually impossible to make firm statements about what minimum dose of ionizing radiation is absolutely harmless, we try to take whatever steps are available to us to reasonably reduce exposure. Sealing the x-ray room in the bottom of an abandoned mine would minimize public radiation exposure, but be prohibitively costly and offer only a small reduction in dose. Closing the door to the room offers a probably-comparable protection, but at virtually zero cost — so it is required.
- The rules are usually designed to consider 'worst-case' scenarios, in addition to normal operations. What happens if a resident is just testing the machine (or is teaching students) and happens to have the cone pointed at a weird angle? What happens if someone moves or replaces some of the furniture in the room, so that the scattering geometry becomes more favorable? What happens if the cleaning lady, receptionist, lost patient, or a new dentist on staff assumes that it's okay to enter the room since the door is wide open? If you only leave the door open when you're sure that the beam isn't pointed at the door, are you certain that you've remembered to close it every single other time? If there are a few situations where leaving the door open might be dangerous, but none where it would be harmful to have it closed, then it may be reasonable to require that the door be closed at all times. TenOfAllTrades(talk) 16:50, 24 August 2009 (UTC)
- Incidentally, the best person to discuss your specific situation with will be your facility's designated Radiation Safety Officer. The exact title differs somewhat by jurisdiction, but there ought to be a specific responsible individual tasked with organizing radiation safety courses, filling out radiation-related paperwork, and all that. Any information that we provide here may or may not apply to your situation; we also cannot comment on specific regulatory requirements in your jurisdiction. Your RSO should be able to tell you how specific local safety requirements were established. TenOfAllTrades(talk) 16:55, 24 August 2009 (UTC)
I'm very impressed with everyone's contribution. Thank you to all and to all a thank you! DRosenbach (Talk | Contribs) 18:39, 24 August 2009 (UTC)
- I'm really surprised that what *I* think is the most important reason for protecting the staff didn't get mentioned until the last response. In fact, I'd say that cumulative exposure may be significant is a significant understatement :-).
- A few shots of dental-grade radiation aren't going to do much damage to anybody; a twice-a-year set plus a full-mouth reference set every 2-3 years for the past 50 years doesn't seem to have hurt me (as near as I can tell). On the other hand, catching a few loose rays 4-6 times a day, five days a week, is an entirely different story.
- --DaHorsesMouth (talk) 23:04, 24 August 2009 (UTC)
- Cumulative exposure is for sure paramount -- but my question is sort of similar to "is standing 100 feet away any better than standing 20 feet away." In the same vein, if no radiation can be detected past the 180 degrees in which the cone exists, why develop methods of precaution that are based on ignorance? DRosenbach (Talk | Contribs) 03:31, 25 August 2009 (UTC)
- Well, sure, the amount of radiation is an inverse-range-squared thing - so at 100 feet, you're getting 1/25th (4%) of the dosage that you would at 20 feet...and 1/10,000th the dosage the patient is getting at 1 foot. But cumulative effects matter - so doing it 10 times a day for 200 days a year gets you 2000 times the dose you'd have gotten from a single shot. The trouble is that the whole thing is statistical. You can get a fatal cancer from one single event where you were standing 100 feet away behind a foot of lead - or you could get X-rayed once a day for your entire life and never suffer at all...but the odds of either of those extremes happening are very small. Since there is no 'safe' dose - the only thing you can do is to minimise the exposure to everyone and calculate the risk/benefit ratio. SteveBaker (talk) 15:54, 25 August 2009 (UTC)
- Cumulative exposure is for sure paramount -- but my question is sort of similar to "is standing 100 feet away any better than standing 20 feet away." In the same vein, if no radiation can be detected past the 180 degrees in which the cone exists, why develop methods of precaution that are based on ignorance? DRosenbach (Talk | Contribs) 03:31, 25 August 2009 (UTC)
- It looks like the OP needs data on the actual stray radiation. The operatory is obviously equipped with X-ray sensitive film and means of developing it. I suppose one could fix pieces of film close to the generator, on the door and midway and compare the results after varied prolonged exposures. Cuddlyable3 (talk) 00:24, 25 August 2009 (UTC)
- It's not my room, so I don't know how well the films will remain when I'm not physically monitoring them, but it's an idea. DRosenbach (Talk | Contribs) 03:31, 25 August 2009 (UTC)
- Everyone who uses the facility on a regular basis should also wear a Film badge dosimeter and have it checked regularly. SteveBaker (talk) 15:57, 25 August 2009 (UTC)
- It's not my room, so I don't know how well the films will remain when I'm not physically monitoring them, but it's an idea. DRosenbach (Talk | Contribs) 03:31, 25 August 2009 (UTC)
- It looks like the OP needs data on the actual stray radiation. The operatory is obviously equipped with X-ray sensitive film and means of developing it. I suppose one could fix pieces of film close to the generator, on the door and midway and compare the results after varied prolonged exposures. Cuddlyable3 (talk) 00:24, 25 August 2009 (UTC)
spontaneous endothermic solvation
[edit]If some solvation reactions are endothermic (e.g. dissolving NaCl in water) ... why do they spontaneously occur? Or does the entropy term become significant in solvation reactions? John Riemann Soong (talk) 18:53, 24 August 2009 (UTC)
- Bingo; you hit the nail on the head. Roughly speaking, as long as the change in the Gibbs free energy (ΔG) is negative, a reaction proceeds spontaneously. Dissolution of a crystalline solid in water is always going to be associated with a big increase in entropy, so the reaction is pushed towards dissolution. TenOfAllTrades(talk) 19:26, 24 August 2009 (UTC)
- Why is the entropy term so significant here? I do notice however, a few things... extra fine sugar is more soluble than if you just dissolved regular coarse sugar.... why would the solubility change here? Also, when solubility is like 20 times greater at 100 C than at 20 C for some substances, is this great solubility change generally applicable only for endothermic solvation? (That is, the heat supplies energy for the solvation?) Does stirring or agitation supply any activation energy for the solvation? John Riemann Soong (talk) 12:44, 25 August 2009 (UTC)
- Extra fine sugar has a higher surface area, due to the smaller grains - that's why it dissolves faster, I'm not sure that it is more soluble - though maybe you are making a supersaturated solution? which is easier with finer grains for the same reason.
- Changes in solubity with temperature - temperature increases favour dissolving (reaction) in an endothermic reaction see [2] also Le Chatelier's principle. Additionally if the entropy increases for a reaction an increase in temperature will tend to make the reaction go further (ie more soluble)
- Stirring supplies heat and work to the mixture - which helps the dissolution, very strong stirring could help break up the solid to help it dissolve, it doesn't really supply activation energy - but does help speed up equilibrium by mixing. In an un stirred mixture of sugar or salt in water the solid crystals will become surrounded by a concentrated solution of the same substance - which prevents further dissolution - in this case the dissolving is limited by diffusion of the stronger solution around the crystals into the whole of the liquid. Stirring overcomes this slow diffusion limiting step by constantly diluting the liquid around the crystals. 83.100.250.79 (talk) 14:01, 25 August 2009 (UTC)
- Alright, that means the (metastable) solubility won't actually decrease after lowering the solution back to RT, right? Actually I've been suspecting this for some time -- I would would dissolve a heck a lot of sugar into hot boiling water, bring it back to RT (to make custard) and wonder why I didn't observe any sugar dropping out of solution. (Yet however, if I do this for hot tea or coffee, I will notice sugar inevitably dropping out of solution as the tea/coffee cools.) For a while I had this impression that increased temperature created greater interstitial gaps between solvent molecules or some other structural effect (thermal expansion) happened at high temperatures and this led to increased solubility. John Riemann Soong (talk) 15:13, 25 August 2009 (UTC)
- Nope, increased temperature doesn't create "interstitial gaps" (at least not to any appreciable extent), what it does is (in lay terms) make the solvated molecules bounce around more and thus make them less likely to stick together and fall out of solution. What you were doing in the custard example was to create a supersaturated solution of sugar -- the sugar concentration was actually more than the solubility limit, but there was nothing there to induce crystallization. Whereas in tea or coffee, you got a whole bunch of other compounds floating around, and some of them may lower the solubility of sugar and/or promote crystallization. 98.234.126.251 (talk) 04:59, 26 August 2009 (UTC)
- Custard may stabilise a supersaturated solution, it may bind to either salt or sugar (via donor, or hydrogen bonds). Also it may serve to trap very small crystals of sugar that have come out of solution - in fact the custard may contain a fine suspension of sugar/salt stabilised by the custard which prevents it from crystallising out in the normal way.83.100.250.79 (talk) 12:53, 26 August 2009 (UTC)
- Hmmm, later I mix in eggs and milk, and don't notice any sugar dropping out of solution either ... but then again it might be suspended in proteins, albumen and milk fat as the mixture thickens. Basically, what I'm wondering is whether the main mechanism of the added heat is to supply the free energy required for endothermic solvation, or whether it's a kinetic effect...that is, when you lower it to RT, it takes a while for the free energy of the solvated molecules to "decay" and reverse the reaction. John Riemann Soong (talk) 15:53, 26 August 2009 (UTC)
- You may wonder, but I doubt anyone knows, it may be both... Note we don't know how much custard, sugar etc you are using, and even if we did, we would probably have very much difficulty finding thermodynamic data to answer your question conclusively...83.100.250.79 (talk) 19:22, 26 August 2009 (UTC)
- For sugar in water? Well I do wonder if the possibility that increased temperature provides the enthalpy for some of the heat of solvation would explain the stability of supersaturated sugar solution. Basically, the idea is that solubility changes aren't symmetrically reversible -- the solubility of say some solute in water may increase 20-fold at 100 C, but the solubility will decay slowly when you bring it back to RT. John Riemann Soong (talk) 15:29, 28 August 2009 (UTC)
- You may wonder, but I doubt anyone knows, it may be both... Note we don't know how much custard, sugar etc you are using, and even if we did, we would probably have very much difficulty finding thermodynamic data to answer your question conclusively...83.100.250.79 (talk) 19:22, 26 August 2009 (UTC)
- Nope, increased temperature doesn't create "interstitial gaps" (at least not to any appreciable extent), what it does is (in lay terms) make the solvated molecules bounce around more and thus make them less likely to stick together and fall out of solution. What you were doing in the custard example was to create a supersaturated solution of sugar -- the sugar concentration was actually more than the solubility limit, but there was nothing there to induce crystallization. Whereas in tea or coffee, you got a whole bunch of other compounds floating around, and some of them may lower the solubility of sugar and/or promote crystallization. 98.234.126.251 (talk) 04:59, 26 August 2009 (UTC)
- Also, do we observe the opposite effect -- that temperature increase decreases solubility of say, HCl in water? John Riemann Soong (talk) 17:39, 25 August 2009 (UTC)
- That's because HCl is a gas, and therefore comes out of solution at increased temperatures. 98.234.126.251 (talk) 04:50, 26 August 2009 (UTC)
- But HCl completely dissociates in water ... how would one distinguish between heat reversing an exothermic reaction and increased vapor pressure of the solutes? John Riemann Soong (talk) 15:53, 26 August 2009 (UTC)
- HCl is a special case since it is a gas , and can evaporate, heating it up tends to make it boil off (despite the dissociation) - the effect is common to all dissolved gases. Effectively distillation of the HCl is competing.83.100.250.79 (talk) 21:37, 26 August 2009 (UTC)
- But HCl completely dissociates in water ... how would one distinguish between heat reversing an exothermic reaction and increased vapor pressure of the solutes? John Riemann Soong (talk) 15:53, 26 August 2009 (UTC)
- According to [3] soldium sulphate is an example of something that decreases in solubility with temperature. See Sodium sulphate
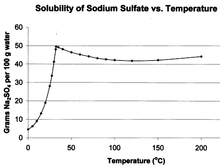
Na2SO4 solubility curve - In general such behaviour is rare, due to the almost guaranteed increase in entropy on dissolution.
- Also see my question below Wikipedia:Reference_desk#Sodium Sulphate solubity 83.100.250.79 (talk) 21:32, 26 August 2009 (UTC)
- That's because HCl is a gas, and therefore comes out of solution at increased temperatures. 98.234.126.251 (talk) 04:50, 26 August 2009 (UTC)
- Alright, that means the (metastable) solubility won't actually decrease after lowering the solution back to RT, right? Actually I've been suspecting this for some time -- I would would dissolve a heck a lot of sugar into hot boiling water, bring it back to RT (to make custard) and wonder why I didn't observe any sugar dropping out of solution. (Yet however, if I do this for hot tea or coffee, I will notice sugar inevitably dropping out of solution as the tea/coffee cools.) For a while I had this impression that increased temperature created greater interstitial gaps between solvent molecules or some other structural effect (thermal expansion) happened at high temperatures and this led to increased solubility. John Riemann Soong (talk) 15:13, 25 August 2009 (UTC)
- Why is the entropy term so significant here? I do notice however, a few things... extra fine sugar is more soluble than if you just dissolved regular coarse sugar.... why would the solubility change here? Also, when solubility is like 20 times greater at 100 C than at 20 C for some substances, is this great solubility change generally applicable only for endothermic solvation? (That is, the heat supplies energy for the solvation?) Does stirring or agitation supply any activation energy for the solvation? John Riemann Soong (talk) 12:44, 25 August 2009 (UTC)
fm transmitter using crystal oscilator119.152.15.223 (talk) 18:56, 24 August 2009 (UTC)
[edit]HY AGAIN ITS KIND OF A SILLY QUESTION BUT I CANT HELP CAN I MAKE FM TRANSMITTER USING CRYSTAL(ONE USED IN CONTROLLERS) ONLY AND NO INDUCTOR? ALSO I URGENTLY NEED A FREE FM TRANSMITTER DESIGN BOOK(UPDATED AND UPTODATE)THAT WOULD MAKE ME DESIGN A CIRCUIT MYSELF .....I HAVE TRIED WEBSITES BUT THEY CONTAIN RANDOM MATERIALS...PLZ HELP ME!!!—Preceding unsigned comment added by 119.152.15.223 (talk • contribs)
- Please do not post in all caps, because it is the web equivalent of shouting, and please sign your posts with four tildes ~~~~. Were you planning on using integrated circuits, transistors, or tubes in your transmitter in addition to the crystal? What do you have against inductors, which for radio purposes can be made with a bit of wire? Edison (talk) 19:01, 24 August 2009 (UTC)
- While crystals make a good oscillator, they usually need some capacitors at the output taps to tune the resonance. Also, the signals tend to be really weak, so you'll probably want an amplifier as well. You can get away without an inductor, (at least, a lumped-element version) - but it depends on your signal quality needs. The biggest question is, do you have test equipment? It's going to be hard to verify that you're actually oscillating, especially if you're playing games with passive tanks and no amplifier, unless you have a pretty good oscilloscope or a spectrum analyzer. As far as free circuit designs, have you looked on the web? I just searched Google, and found a single-transistor VCO (varactor, not crystal) which might suit your needs. What are you looking for? Do you have a frequency range in mind? Do you want tunability? How accurate are your frequency requirements? What are your power, noise, and bandwidth requirements? Do you want to build it entirely from discrete components? You should think about these questions before you dive into the electronics project. If you don't know the answers to these questions (in fact, if you don't know what they mean), you might consider scaling back your design quite significantly - building a wireless transmitter from scratch can be a pretty heavy-duty and difficult hobby project. You might want to think about buying a commercially-available, off-the-shelf TR unit. Here are over 1,000 available types from Digikey. I found some as low as $1 and $2 US. There is no way you can beat those prices if you're building out of discrete components. Nimur (talk) 21:02, 24 August 2009 (UTC)
- Here is a cheap ready-made FM transmitter. If you are thinking of making a Voltage-controlled crystal oscillator they can in principle generate an FM signal but its frequency can deviate too little from the nominal crystal frequency to be much use, and the centre frequency is fixed. A crystal oscillator without an inductor must operate at a fundamental crystal frequency e.g. 1 to 20 MHz because a tuned circuit with an inductor would be needed to select a higher harmonic frequency. Perhaps you should forget about using a crystal and instead make, say, a simple LC oscillator, establish that it produces a signal (how? ideally using a spectrum analyzer or an oscilloscope, or you may detect some interference on a nearby radio) to which you add varicap diodes so the frequency can be modulated. That is like the bottom circuit at the reference that Nimur provided except you disconnect from ground the 1µF capacitor shown at the terminal labelled PLL IN (optional) and instead feed your audio signal to the lower end of the capacitor. Cuddlyable3 (talk) 00:07, 25 August 2009 (UTC)
siphon question
[edit]I know you can siphon liquid from one container to another, can you do the same thing with gas (the state of matter, not the fuel)? I imagine that the cohesion of the liquid droplets from hydrostatic forces is contributing, but is there a similar driver in a gas such as steam? Googlemeister (talk) 20:32, 24 August 2009 (UTC)
- To a first approximation, there is no attractive forces between gas molecules, see Kinetic Molecular Theory and Ideal gas. However under certain extreme conditions this approximation breaks down measurably, see Van der Waals equation. I don't, however, think that under normal conditions a syphon will work for a gas any faster than normal diffusion would. --Jayron32 20:40, 24 August 2009 (UTC)
- I would think it might work if you had a very heavy gas (much heavier than the surrounding environment). Just as in a liquid siphon tube, you'd need to avoid getting air bubbles in the tube. This might be tougher than with a liquid, though, as you'd need both ends of the tube to be fully submerged in the heavy gas at all times. Lifting even the downstream end out of the heavier gas briefly would likely fill it with air and break the siphon. StuRat (talk) 21:18, 24 August 2009 (UTC)
- I think it'll work just fine with a gas. Pick a heavier than air gas and pretend it's water. It doesn't require "cohesiveness" or whatever. The pressure in one end of the siphon tube is higher than the other...gas flows from high to low pressure. The gas doesn't "know" that it's in a siphon tube...it could be in a pressurized natural gas pipeline goind over a hill or something. Also, being very much lighter than most liquids, the siphon tube could be very tall before it would cavitate (Mercury: 0.7 meters or so, Water: 10-ish meters, CO2: A kilometer or so maybe?)...presumably, you could even use a lighter gas - two inverted fish-bowls full of helium with a U-shaped tube between them should allow helium to flow from the lower bowl to the higher one. SteveBaker (talk) 22:35, 24 August 2009 (UTC)
- Sulfur hexafluoride is a gas that is sometimes used for these kinds of experiments because it is non flammable and non toxic and very heavy for a gas. You can find vidoes online of an experiment of a paper boat floating in a fish tank half full of SHF. It looks just about how you'd expect it to look if it was floating on water, except the tank looks empty. I agree with what Stu has said about the both ends needing to be submerged. With a liquid, the liquid density is sufficient to stop air climbing up the hose and breakind the syphon, but with gas, the density isn't there and I think air would get into the end of the hose. Vespine (talk) 22:51, 24 August 2009 (UTC)
- Our siphon article asserts that it works exactly because of the cohesiveness, and not because of the pressure difference between the source and the sink. --Sean 23:52, 24 August 2009 (UTC)
- No, it doesn’t. The opening sentence says “…the flow being driven only by the difference in hydrostatic pressure…”, and Siphon#Explanation using Bernoulli's equation involves only pressure. “Cohesiveness”, i.e., tensile strength, is only mentioned in the Siphon#Vacuum siphons section as being a secondary effect, that can overcome the normal height limit imposed by purely pressure considerations. Red Act (talk) 00:46, 25 August 2009 (UTC)
- I think it'll work just fine with a gas. Pick a heavier than air gas and pretend it's water. It doesn't require "cohesiveness" or whatever. The pressure in one end of the siphon tube is higher than the other...gas flows from high to low pressure. The gas doesn't "know" that it's in a siphon tube...it could be in a pressurized natural gas pipeline goind over a hill or something. Also, being very much lighter than most liquids, the siphon tube could be very tall before it would cavitate (Mercury: 0.7 meters or so, Water: 10-ish meters, CO2: A kilometer or so maybe?)...presumably, you could even use a lighter gas - two inverted fish-bowls full of helium with a U-shaped tube between them should allow helium to flow from the lower bowl to the higher one. SteveBaker (talk) 22:35, 24 August 2009 (UTC)
- Yes, it does:
- Liquids can rise over the crest of a siphon because gravity pulls on the greater weight of the liquid in the longer outlet leg ... A common misunderstanding of siphons is that atmospheric pressure is pushing the liquid over the barrier. This is easily disproved by noting that atmospheric pressure pushes equally on the surface of the liquid at both the inlet and outlet of the siphon. Thus atmospheric pressure contributes no net force on the liquid in either flow direction.
- Emphasis mine, but obviously if there's pulling going on it needs cohesion. They go on to give an analogy about trains where the train couplings represent the liquid's cohesiveness. I believe the difference in hydrostatic pressure they're referring to is the low pressure caused by the pulling/evacuation, not the difference in air pressure at the surface of the two reservoirs, which would cause pushing, and is lower on the high reservoir in any case. --Sean 12:54, 25 August 2009 (UTC)
- Yes, it does:
- The difference in pressure is due to the downstream tube having more length pointing downward, which means more gas and mass being pulled downwards by gravity. So, if a gas siphon wouldn't work, as you claim, what do you say would happen ? Would you get a vacuum in the tube ? Would the tube remain filled with gas but just refuse to flow ? StuRat (talk) 15:02, 25 August 2009 (UTC)
- It’s not true that “if there’s pulling going on it needs cohesion”, when it’s gravity that’s doing the pulling. If you drop an object in a vacuum chamber, gravity will “pull” it toward the earth, even though there’s no material providing cohesion in between the object and the Earth.
- Yes, it’s true that atmospheric pressure isn’t what causes the net force on the liquid. There is, however, a difference in hydrostatic pressure between the source and the sink, due to the greater weight of the liquid in the longer outlet leg. Namely, the difference in pressure due to the weight of the liquid is , where is the density of the liquid, is the gravitational acceleration, and is as on the diagram in the siphon article.
- The cohesion of the liquid is only important in that it prevents the liquid from changing to a very low density state, not because the cohesion is what conveys the force in the siphon (or not mainly, anyway). The main thing that conveys the force is the pressure. The couplings on the train analogy really just confuse the issue, and make for somewhat of a bad analogy. A better analogy would be to discard the couplings between the cars, letting the cars just push against each other but never pull, and put an engine on both sides of the train, pushing inward, to represent the air pressure on both ends of the siphon. The two engines push inward with equal force, so provide no net force on the train. But the extra weight of the cars on the longer side of the hill overcome some of the force provided by the lower engine, so the net force on the train is toward the lower side. But there is no net flow at all if the upper engine doesn’t provide enough force to push the trains on its side of the hill to the top, which is analogous to the maximum height of the siphon being when the atmospheric pressure just balances the pressure Red Act (talk) 15:29, 25 August 2009 (UTC)
- P.S. Some actual numbers may be useful here to show that it’s pressure, not cohesion, that’s of primary importance in a siphon (unless the siphon is extremely thin, such that capillary action dominates). According to the surface energy article, the energy of cohesion per unit area of a substance is twice the substance’s surface energy per unit area. That article lists the surface energy per area of water as being 0.072 J/m2, which means that water’s energy of cohesion per area is 0.144 J/m2. 0.144 J/m2 is only enough to lift water by about 3.8mm. Think about a water droplet forming. The droplet can only get about 3.8mm high before gravity overpowers the cohesion of the water, and the droplet falls away. In contrast, one atmosphere (unit) is enough to lift water by about 1033cm. So one atmosphere of pressure is about 2700 times as strong as the water’s cohesion. That’s why when showing the calculation for the maximum height of a siphon, the siphon article just takes atmospheric pressure into consideration, and completely neglects the effect of the liquid’s cohesion. Red Act (talk) 17:46, 25 August 2009 (UTC)
- Thanks for those numbers, they really help to settle the argument. StuRat (talk) 15:23, 27 August 2009 (UTC)
Dr Jeffrey Arnett's theory of Emerging adulthood
[edit]How well accepted is this theory in the scientific community? It seems that this theory focuses only on opinions of perceptions of people in that age group. Also does this theory not contradict the concept of adolescence (the transitional phase between childhood and adulthood)? It seems to me that this proposed stage is a transitional stage between a transitional stage and the resulting stage. His theory seems to propose that Emerging adulthood is a transitional stage from adolescence to adulthood. It also seems that this proposed stage of emerging adulthood is longer than adolescence itself. 86.140.47.91 (talk) 23:12, 24 August 2009 (UTC)
- Here's how to figure out how much attention the idea is getting: go to Google Scholar, and search for "Jeffrey Arnett Emerging adulthood". Look at the top entry, and you see "Cited by 1226". That means that the paper has been cited by 1226 academic publications, a huge number. The idea may or may not be widely accepted, but it's definitely getting a lot of attention. You can click on the "cited by" link to get a list of the papers that cite it, in order of "google weight". Looie496 (talk) 02:28, 25 August 2009 (UTC)






