Wikipedia:Reference desk/Archives/Science/2007 August 31
| Science desk | ||
|---|---|---|
| < August 30 | << Jul | August | Sep >> | September 1 > |
| Welcome to the Wikipedia Science Reference Desk Archives |
|---|
| The page you are currently viewing is an archive page. While you can leave answers for any questions shown below, please ask new questions on one of the current reference desk pages. |
August 31
[edit]Have just been reading this article, and am finding it really difficult to wrap my head around. I can understand all the other universe expansion, theory of relativity things (was always very good at physics) but I'm hoping someone can give me a simple analogy for the Fourth dimension.I'm looking at my monitor, it has an "arrow" for up (height) and arrow away from me (depth) and an arrow running across my (width). With time as the Fourth dimension, does this mean it has another arrow pointing somewhere else representing this progress of the monitor through time (progressing along with me)? If so, where is this arrow pointing? The "time-vectors" of all objects must be the same length otherwise we would all be jumping through time at different speeds? what?! huh? Help! SGGH speak! 00:17, 31 August 2007 (UTC)
- Thinking of time as a fourth dimension is very hard to wrap one's mind around. Instead, try to imagine time as the third dimension in some imaginary 2 dimensional universe. In this universe, everything is only described as width and depth, there is no height. Imagine also that this universe is finite in size, shaped like a square. You are looking at it now. Any given moment in this universe can be captured in a 2 dimensional image of the universe, like individual frames of a video. But instead of playing the frames continuously to observe the universe in motion, stack the frames on top of one another. You have now introduced height, where height is time. Any particle that remains motionless is a straight, vertical line in in your 3D space-time. If an object is in motion, it will trace out a more complex curve through this 3D space-time. If you slice out any cross section of this space-time, you have captured for yourself a freeze-frame of the universe in a given moment. I hope that gives you some guidance. Someguy1221 00:31, 31 August 2007 (UTC)
- that is actually really helpful, thankyou! So in essence, time's 4th dimension is the 3D universe "stacked" ontop of itself for each frame? And the movement of these complex 3D shapes would produce some very complex curves indeed if you managed to link them all together dot-to-dot and view the universe kind of... "side on" if we use that 3D square model of yours. So in theory, as height in your 3D model has a vector and a direction, time has a vector and a direction? Does this mean time has a direction of travel that is relative to length width and depth? If an object could somehow move through the frames quicker than other objects, would it have a larger vector? Or have I extended your model too far? I'm basially asking if time as a front, like your length and width and depth has finite ends, is an object at the end of its "time" at all times because it is in the present, just as the edge of my tv screen is at the end of its length/width/depth? SGGH speak! 00:48, 31 August 2007 (UTC)
- I'm not sure how to answer all of those, but I'm sure someone else will come along soon. Time is always pointing straight up in the example I provided. The directional change is in the total "space-time" vector. As for the "ends" of a particle's extent through time, the only ends would be wherever the particle is created or destroyed, otherwise it will continue on through the time dimension (unless time itself ends at some point). You may be interested in reading Minkowski diagram, a scientifically rigorous method of diagraming 2D space-time. Someguy1221 01:06, 31 August 2007 (UTC)
- The traces of a 4-dimensional object in three-space are solids. It's possible for a single 4-dimensional object to create multiple solid objects as the traces in 3D, just as a single 3D object (say an octopus) can create multiple 2D curves (cross sections) in any 2D plane. StuRat 03:12, 31 August 2007 (UTC)
- It's worth noting that there are different types of "4 dimensional space" - spacetime mentioned above is one, another is simply the addition of a fourth orthogonal axis to the 3 (length )dimensions most people experience - see Fourth dimension. These are often called 'einsteinian' and 'spatial' fourth dimensions respectively.213.249.232.202 06:06, 31 August 2007 (UTC)
- "Most people"? Are there cases of people who think they live in a 4D world? Does WP have articles on them? Aaadddaaammm 09:05, 31 August 2007 (UTC)
- "...the 3 (length )dimensions most people experience " 3, three...????????213.249.232.202 09:46, 31 August 2007 (UTC)
- "Most people"? Are there cases of people who think they live in a 4D world? Does WP have articles on them? Aaadddaaammm 09:05, 31 August 2007 (UTC)
- It's worth noting that there are different types of "4 dimensional space" - spacetime mentioned above is one, another is simply the addition of a fourth orthogonal axis to the 3 (length )dimensions most people experience - see Fourth dimension. These are often called 'einsteinian' and 'spatial' fourth dimensions respectively.213.249.232.202 06:06, 31 August 2007 (UTC)
- Sadly, almost everyone seems to fail to understand what 4 dimensional space means. Maybe an elementary course in linear algebra could help, but, in the meantime, don't panic: 4 dimensions means having "4 variables" in which to store information, nothing more, nothing less. Living in a world of 4 dimensions means we have 4 variables attached to every object in our universe, 3 for what we call "space" altogether and one for time. We could imagine a world of 12 dimensions too, just think of having 3 for space, one for time, one for its angular momentum, another for its favourite football team... --Taraborn 10:38, 31 August 2007 (UTC)
- Actually the title article Fourth dimension describes four dimensional space (not einsteins space time) 'quite well'?87.102.88.202 13:57, 31 August 2007 (UTC) I can't find copies of equations for rotations etc in four spatial dimensions on wikipedia - maybe are expected to work those out for ourselfs?87.102.88.202 14:07, 31 August 2007 (UTC)
- It's better to use linear algebra for this. Represent positions as vectors, represent rotations as vectors. The 'equation' for N-dimensional rotation then becomes nothing more than an N-dimensional matrix multiplication which is easy to understand for any value of 'N'. So take some time to soak up some 3D linear algebra. Once you've seen how to make a rotation matrix in 3D and how to multiply matrices in 3D, the extension to 4D is obvious and natural. Incidentally, in the world of computer graphics, 4D matrix arithmetic is what we use all the time for doing 3D graphics because it allows translations and perspective calculations to happen naturally and at the same time as rotations. Hence computer graphics hardware already works in 4D! SteveBaker 15:16, 31 August 2007 (UTC)
- Ooops sorry - I alreagy had that (interesting to consider rotating about planes etc - but impossible for me to visualise) - I've caused you unneccessary typing - I meant that wikipedia doesn't have the answers worked out for us for higher dimensional transformations (can't find the page and imagine it doesn't exist) - sort of rhetorical question but not very well worded. Perhaps such a page should exist?
- (Thinking about it aren't modern GPU's set up specifically for 3d - in terms of their parallelism - for quickly performing projections and rotations etc - maybe they wouldn't work so well with more parameters because the instr set doesn't support it - trivia)
- It's better to use linear algebra for this. Represent positions as vectors, represent rotations as vectors. The 'equation' for N-dimensional rotation then becomes nothing more than an N-dimensional matrix multiplication which is easy to understand for any value of 'N'. So take some time to soak up some 3D linear algebra. Once you've seen how to make a rotation matrix in 3D and how to multiply matrices in 3D, the extension to 4D is obvious and natural. Incidentally, in the world of computer graphics, 4D matrix arithmetic is what we use all the time for doing 3D graphics because it allows translations and perspective calculations to happen naturally and at the same time as rotations. Hence computer graphics hardware already works in 4D! SteveBaker 15:16, 31 August 2007 (UTC)
- Actually the title article Fourth dimension describes four dimensional space (not einsteins space time) 'quite well'?87.102.88.202 13:57, 31 August 2007 (UTC) I can't find copies of equations for rotations etc in four spatial dimensions on wikipedia - maybe are expected to work those out for ourselfs?87.102.88.202 14:07, 31 August 2007 (UTC)
87.102.88.202 16:03, 31 August 2007 (UTC)
- (Oh I guess you meant the VMX type instructions with 4 32 bit values per register - yes that could be useful for 4D)87.102.88.202 16:06, 31 August 2007 (UTC)
- Firstly, it is not at all clear that time should be considered to be a 'fourth dimension' that can be treated as space is. There are many time-effects (such as entropy and our human perception of time) that are quite unlike any effect we see happening in the three space-like dimensions. So we should really consider 'time-like dimensions' and 'space-like dimensions' separately. When you do that, the question of how the world would look if it had four space-like dimensions is the real question - and it is a tricky one.
- An important first part of the question is: "How does 3D space look?" - you may think you know the answer but you don't. Our eyes are only 2D devices - we see images projected onto a 2D retina and our brains somehow manage to interpret this as a 3D world. (People will argue that we have two eyes and stereo effects let us see in 3D but that's bogus for a lot of reasons - close one eye and the world still looks pretty much the same). So what we actually see is a PROJECTION of the 3D world onto our 2D retinas. If you want to know what a 4D world would look like when projected onto our 2D retinas - just write a computer program to do that. It's easy. There are a bunch of them out on the Internet. Here is a game that you can play in 2, 3 or 4 dimensions.
- Are there people who think they live in a 4D world? Oh yes! There are string theorists who believe that we live in a 26 dimensional world. Do these people SEE in more than 3 dimensions? No - they still have 2D retinas just like you and me. If the universe had more than three 'ordinary' space-like dimensions then photons would move in all four dimensions and the world would look like it does in that 4D video game - and we'd see that and would have evolved to understand it too.
- Since that's not the case, then any 'extra' dimensions have to be in some way different from the three that we're familiar with. Well, we have that one 'time-like' dimension - and that's definitely different. The string theorists claim that ever since the big bang, their twenty-odd extra dimensions have been 'curled up' tightly - so (for example) the 4th dimension is such that if you move along it for a short distance, you end up back where you started. (Kinda like flying around the world and coming back to where you started). But that 'short distance' is insanely short - much MUCH shorter than the diameter of an atom. That being the case, anything that was moving or placed 'off to one side' in that extra dimension would look exactly like it was right here in the same place as us along that fourth axis. You can't turn your head by 90 degrees and face along the 4th dimension because there isn't enough room! Extend that out to 20-some extra dimensions and things still look pretty much like a 3D world.
- So if you believe the string theorists (and I'm not sure that I do) - then you are ALREADY seeing perfectly well in a 26 dimensional world - and it looks like you think it does!
- SteveBaker 14:13, 31 August 2007 (UTC)
- Is it possible that a 4 or higher dimensional (spatial) being could 'project' themselves into 3 dimensional space?87.102.88.202 14:45, 31 August 2007 (UTC)
- That question is meaningless. If there were four (or more) 'unwrapped' space-like dimensions then we'd see in 4D and we'd be 4-dimensional-beings ourselves. You can't have 4-dimensional beings without there being 4-dimensional space...and there isn't, so there isn't. You can't have some things in the universe having a different number of dimensions than others because the 'dimensionality' of the universe is a property of the laws of physics - not the nature of a particular object. However, you can write computer software to 'project' a 4D object onto a 2D computer screen - and (as I said) there are plenty of places on the net where you can see that. It's mostly just confusing - not particularly mind-blowing at all. But check it out for yourself here, for example. SteveBaker 14:59, 31 August 2007 (UTC)
- Those are confusing in part because they are projecting a 4D idea into a 3D space represented on a 2D monitor. An easier projection is the one we actually see: things change over time, though we only see one discrete moment of it at a time. It is easy to describe an ice cube in 4 dimensions. The 3 spatial dimensions are simple — it is a cube, after all — and then 4-D description is just its decay from time t to time t'. What we can't easily do is see the entire "lenght" of the fourth dimension at any single time. We could superimpose all images of the ice cube on top of itself, but that would look like a jumble, in the same way a hypercube does. We are 4-dimensional beings — we age, we move through spacetime. If we were only 3-dimensional beings it would look frozen, static. --24.147.86.187 23:05, 31 August 2007 (UTC)
- Yes - but our eyes ARE 2D image receptors - just like the computer monitor. The question is about what a 4D world would look like to human eyes - so a projection down to 2D is precisely the right answer. Now, you might ask what a 4D world would look like to people with 3D optical recptors in their 4D eyes - and that's something we can't know. Mapping the extra dimension into time (as you advocate) doesn't help at all! If you map a common 3D object into a series of time-wise 2D cross-sections, you can't grasp the shape of the darned thing at all! That technique gives you no idea whatever what a 4D world would look like. So I'll absolutely defend my previous answer - projecting 4D into 2D lets you see EXACTLY what things would look like when viewed with human eyes in a 4D world. This isn't a speculative or approximate thing at all - we can easily simulate the paths of photons in a 4D world and figure out where each one would intercept a human retina...that's an exact thing and it's what the examples I gave are (essentially) doing. SteveBaker 21:04, 1 September 2007 (UTC)
- Those are confusing in part because they are projecting a 4D idea into a 3D space represented on a 2D monitor. An easier projection is the one we actually see: things change over time, though we only see one discrete moment of it at a time. It is easy to describe an ice cube in 4 dimensions. The 3 spatial dimensions are simple — it is a cube, after all — and then 4-D description is just its decay from time t to time t'. What we can't easily do is see the entire "lenght" of the fourth dimension at any single time. We could superimpose all images of the ice cube on top of itself, but that would look like a jumble, in the same way a hypercube does. We are 4-dimensional beings — we age, we move through spacetime. If we were only 3-dimensional beings it would look frozen, static. --24.147.86.187 23:05, 31 August 2007 (UTC)
- That question is meaningless. If there were four (or more) 'unwrapped' space-like dimensions then we'd see in 4D and we'd be 4-dimensional-beings ourselves. You can't have 4-dimensional beings without there being 4-dimensional space...and there isn't, so there isn't. You can't have some things in the universe having a different number of dimensions than others because the 'dimensionality' of the universe is a property of the laws of physics - not the nature of a particular object. However, you can write computer software to 'project' a 4D object onto a 2D computer screen - and (as I said) there are plenty of places on the net where you can see that. It's mostly just confusing - not particularly mind-blowing at all. But check it out for yourself here, for example. SteveBaker 14:59, 31 August 2007 (UTC)
- Is it possible that a 4 or higher dimensional (spatial) being could 'project' themselves into 3 dimensional space?87.102.88.202 14:45, 31 August 2007 (UTC)
- What I find odd is that there is no good reason why we only have 3 dimensions - 4 or more is possible - as you know. But the universe appears to only have three spatial dimensions - am I missing something obvious as a reason - or is this a good example of an unanswered question in physics/philosophy - "why 3 spatial dimensions" (I release that answers such as "because it is - that's what we see etc" are meaningfull - but I'd like to go beyond 'it is').Your comments appreciated.87.102.88.202 15:19, 31 August 2007 (UTC)
- Why 3 dimensions? Well, it's not clear that there are only three. String theorists postulate many more than that. However, if the number is three then I suggest that it's because if there were fewer, then things like atoms could not exist and we wouldn't be here to observe it. This is an appeal to the anthropic principle - but it's the best explanation we have for that. Perhaps (as string theorists suggest) the unnecessary dimensions 'curled up' and collapsed in the first nanoseconds of the big bang and three is some kind of minimum energy configuration or some such. Asking this is a bit like asking why the charge on the electron is what it is - it has to have some value and this happens to be it. SteveBaker 20:55, 1 September 2007 (UTC)
- An appeal to the anthropic principle works for me, as does common sense! but it's still a mystery on a 'cosmoslogical scale' - but then everything else is as well.. Maybe it's a better question for philosophers than scientists - I think it's an unfair question to ask a scientist as they/we have no evidence any other way (or even the ability to check!)..83.100.249.228 21:11, 1 September 2007 (UTC)
- I suggest you read Flatland, which is both entertaining and educational for thinking about the spacial dimensions. -- JSBillings 16:47, 31 August 2007 (UTC)
- I don't think Flatland is any good at all. It talks very little about the nature of spatial dimensions and gets things wrong MANY times (it's description of a 2D house confuses whether it's a sideways cross-section or a plan-view of a 3D building. It's mostly a painfully sexist diatriabe against the nature of class in our society...nothing enlightening about that at all! There are better books about 2D worlds - and the one that rises head-and-shoulders about the others is The Planiverse. SteveBaker 20:55, 1 September 2007 (UTC)
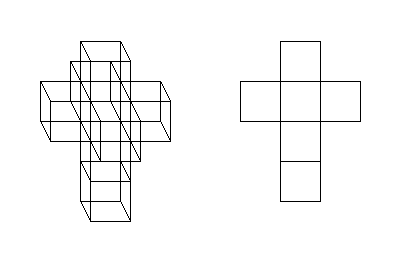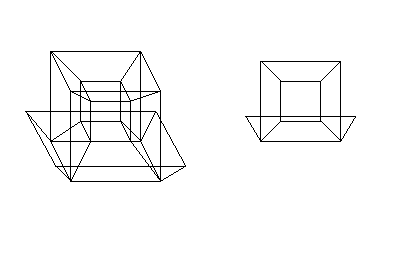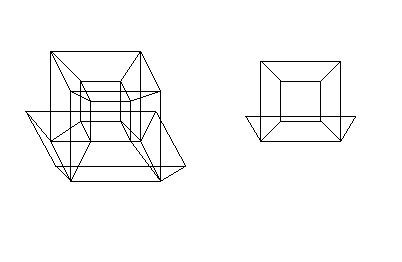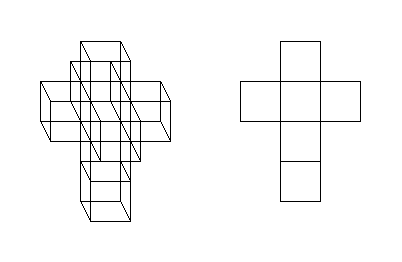
- Hypercube has been mentioned, but not linked to yet. So here you go. :) And here's a more elaborate visualisation of it. DirkvdM 05:17, 1 September 2007 (UTC)
space time-Einstein considered time as a dimension, just like length, width, and height. The 4 dimensions in Einstein physics are called space-time. Even though we can move around freely in 3 dimensions, we're sort of stuck in the 4th one, time. We're all moving forward, aging, along the time dimension. If your brain is hurting, think of it this way: the universe from 1 second ago still exists. You from 1 second ago still exists: you just don't have the ability to move back and forth through time to check it out[source=Popular Science Almanac for kids]--WikiPoTechizen 10:14, 3 September 2007 (UTC)
Blood Sugar Dropping
[edit]Why does blood sugar drop in normal people sometimes and cause them to get dizzy and sometimes faint? I thought when you blood sugar drops and you dont eat, you start breaking down fat. How come the same people dont have the same problem when they exercise? —Preceding unsigned comment added by 76.167.145.55 (talk) 03:13, 31 August 2007 (UTC)
- Please see hypoglycemia, Neuroglycopenia, and maybe even Idiopathic postprandial syndrome. In short, while most of your body can burn other fuel sources, your brain really doesn't like to (I haven't figured this one out yet, but Wikipedia claims it is not fully understood). Someguy1221 03:39, 31 August 2007 (UTC)
- Blood sugars below 80 are usually a signal of some underlying condition. The body's normal response to low blood sugar is to release more sugar into the blood (mainly from the liver I believe). The system that controls storage is usually activated when blood sugar rises above 120. So normal bodies store sugar in the blood when it is more concentrated then 120 and release sugar when the concentration falls below 80. If the sugar level gets low enough, cells start breaking down energy sources other than blood sugar (like the fat you mentioned). However, there is not much fat in and around the brain. There is not much energy storage period above the neck (no protein stores or fat stores). So, blood is the brain's only source of energy. This is why low blood sugar immediately causes symptoms in the brain. Not to mention the brain uses more sugar than than most parts of the body. So when something goes wrong with the release mechanism, the brain suffers because it needs that sugar. Fat, protein, or carbohydrates call all be catabolized into molecules the brain can use for energy. However, this process does not happen in the brain itself.
As for why problems don't happen when a person with chronic hypoglycemia exercises, I'm pretty sure that the major cause of hypoglycemia is related to self-injected insulin. That is a patient who's storage mechanism is broken injects insulin to trigger the storage mechanism and it stores to much (dropping the level below 80). Or, the second most common cause is that a patient's storage mechanism is very sensitive and overrides the release mechanism in certain circumstances. So, a person who normally gets hypoglycemic goes to exercise, their release mechanism works fine and they don't pass out. However, that same person eats only a candy bar and this sends their storage mechanism into overdrive and their blood sugar plummets because even though their release mechanism may start working, their storage mechanism overpowers it. Mrdeath5493 23:10, 31 August 2007 (UTC)
- Blood sugars below 80 are usually a signal of some underlying condition. The body's normal response to low blood sugar is to release more sugar into the blood (mainly from the liver I believe). The system that controls storage is usually activated when blood sugar rises above 120. So normal bodies store sugar in the blood when it is more concentrated then 120 and release sugar when the concentration falls below 80. If the sugar level gets low enough, cells start breaking down energy sources other than blood sugar (like the fat you mentioned). However, there is not much fat in and around the brain. There is not much energy storage period above the neck (no protein stores or fat stores). So, blood is the brain's only source of energy. This is why low blood sugar immediately causes symptoms in the brain. Not to mention the brain uses more sugar than than most parts of the body. So when something goes wrong with the release mechanism, the brain suffers because it needs that sugar. Fat, protein, or carbohydrates call all be catabolized into molecules the brain can use for energy. However, this process does not happen in the brain itself.
Gauss's law
[edit]Another person seems to think that the field due a point charge Q at distance r (due to coloumb inverse square law) is exactly the same as the field due to a evenly distributed charge of Q on the surface of a sphere (of radius R) when r>R. Exactly the same! Can someone else help persuade them that gauss's law is only an approximation to the field when r>R, (and maybe at the same time show that the approximation becomes better as r/R increase..). (Or maybe you'd like to do the opposite!)213.249.232.202 06:37, 31 August 2007 (UTC) ((Comment added after: I'm assuming here that Gauss's law should be consistent and derivable from Coloumb's law))213.249.232.202 07:29, 31 August 2007 (UTC)
- Have you seen the article Gauss's law? No approximations there. It can be derived exactly from a starting point of an inverse square field strength function. And yes, the electric field outside a spherically symmetric system is identical to that of a point charge located at the origin. (In fact, this can occur even when an inverse square law doesn't apply, such as in general relativity.) Confusing Manifestation 06:56, 31 August 2007 (UTC)
- Have you considered that the charge on the outside of the sphere (relative to the line joining the centre of the sphere and point of measurement) is at an angle to that line - and therefor (due to the circular symmetry about that line) the effective field (due to the charge on that circular band of the sphere) is reduced by a factor of cos(angle between line and points on sphere)? - this means that the true field is always less than the field given by gauss's law (except at infintity). That is the approximation.213.249.232.202 07:19, 31 August 2007 (UTC)
- If you want to prove the equality of the predictions using Coulomb's law, be prepared to write out pages and pages of calculus (you also neglect that some charges are closer or further than the center. A charge being closer than it should has a greater effect on its field at your location than its being further away). But please note that Gauss's law was not derived from Coulomb's law, it was derived from the divergence theorem (proof here (not the original!)). This is pure math, based on arbitrary vector fields. Coulomb's law describes a vector field, so Gauss's law applies. If you seriously doubt this, I truly suggest you pick up an introductory vector calculus book for a more detailed proof (I have been seperated from my own temporarily, or else I would read to you from that). Someguy1221 07:52, 31 August 2007 (UTC)
- Actually, I see that Shell theorem has a nice proof (that Gauss's law and Coulomb's law agree) of this for gravity starting from Newton's law of gravitation (just swap the constants to turn it into coulomb's law). (Oh, and it definately took me a lot more calculus to do my own proof! I think it never occured to me to use law of cosines...) Someguy1221 07:57, 31 August 2007 (UTC)
- Well, well, well I really owe you a debt of gratitude for finding me shell theory. Excellent! I haven't checked it thoroughly but it looks like it simplifies the integration a lot - MANY THANKS! (To you and Newton)
- However looking at the integration
- Have you considered that the charge on the outside of the sphere (relative to the line joining the centre of the sphere and point of measurement) is at an angle to that line - and therefor (due to the circular symmetry about that line) the effective field (due to the charge on that circular band of the sphere) is reduced by a factor of cos(angle between line and points on sphere)? - this means that the true field is always less than the field given by gauss's law (except at infintity). That is the approximation.213.249.232.202 07:19, 31 August 2007 (UTC)
There seems to be a problem since
ʃ r2+s2-R2/s2 ds = ʃ (r2-R2/s2 + 1) ds
Which equals [s - (r2-R)2/3s3] and evaluates to:
- between r+R and r-R
2R - (r-R)/3(r+R)2 + (r+R)/3(r-R)2 ?
Not giving the same result as in the text, have I made a mistake?213.249.232.202 08:33, 31 August 2007 (UTC)
- Your notation is unclear but:
- =
- =
- =
- =
- =
- =
- Which matches the quoted result. Dragons flight 09:00, 31 August 2007 (UTC)
Ah yes thanks. (made a mistake)213.249.232.202 09:05, 31 August 2007 (UTC)
Moths.
[edit]Kind of similar to the fly question above. At night, moths esspecially more than any other bug are attracted into my room with the light on. They are attracted directly to the light and fly around it and try to get as close to the light source as possible. Do they do the same during the day? Just try to get as close to the sun as possible? Or is the sky luminous enough to turn off this behavior? What exactly is the advantage of seeking out light? Capuchin 07:06, 31 August 2007 (UTC)
- See Moth, especially Moth#Attraction to light. Moths are typically nocturnal, so they don't see the Sun, and they navigate by the Moon, so artificial lights confuse them. Confusing Manifestation 07:10, 31 August 2007 (UTC)
Quote " It is immediately apparent that for a spherical Gaussian surface of radius r < R the enclosed charge is zero: hence the net flux is zero and the magnitude of the electric field on the Gaussian surface is also 0"
I think this is not quite right - especially in terms of wording
To my thinking it is immediately apparent that the field at r=0 is zero. However if r<>0 but r<R then the field is non-zero - this is not what the article says. Should there be a change of wording or a disclaimer of the variation from reality?213.249.232.202 07:36, 31 August 2007 (UTC)
- Quite right, actually. See above, and see Shell theorem for proof (the same as Coulomb's law, just swap the constants). Someguy1221 07:57, 31 August 2007 (UTC)
- While "immediately apparent" might be a bit generous, the field IS exactly zero everywhere inside of a spherically symmetric distribution. This can be proven mathematically and is the whole point of the shell theorem. 76.225.157.167 08:05, 31 August 2007 (UTC)
- See talk 2 sections above for my questions about shell theorum.213.249.232.202 08:40, 31 August 2007 (UTC)
- The claim is not that it is apparent that the field is zero inside the shell, but that the enclosed charge is zero, which is obvious: all the charge is concentrated on the spherical shell, that's the point. Gauss's law then allows us to reach the nonobvious conclusion that the field is zero. Algebraist 16:20, 31 August 2007 (UTC)
- I couldn't understand the result from gauss's law - but now I've read the proof at shell theorem everything is clear, but thanks anyway..87.102.88.202 16:46, 31 August 2007 (UTC)
phenomena-defying gravity
[edit]I visited a unique place near Nairobi,a town in Athi River area,we were taken to a hill by locals where they poured water on the steep road.Instead of water going down,it went upward.I put our car in netural,instead of going down it went up the hill.It is a phenomena.
Where can I get the explanation.How can I search Wikipedia?
Best regards.
Nizam Khalfan —Preceding unsigned comment added by 202.163.98.109 (talk) 08:45, 31 August 2007 (UTC)
- It's usually explained as being an optical illusion - ie it looks 'up' but that's just due to the lie of the land. Many places like this exist - see Gravity hill for more details.213.249.232.202 08:59, 31 August 2007 (UTC)
- You could add your place to the list now under Gravity hill#Locations.213.249.232.202 09:02, 31 August 2007 (UTC)
Really Odd Insect
[edit]My husband and I drive a truck over the road and have the opportunity to see some really odd and amazing things sometimes. This trip was no different.
While at the shipper this past Tuesday afternoon in West Valley City, Utah, I saw this "thing" crawling around under one of the customers vans. Upon closer examination, this "thing" was an insect of some kind and I would very much like for someone to give me some help on finding out what it was.
It was about 2 1/2 to 3 inches long, had an orange head, black pinchers or jaws (whatever you want to call them), six legs and its body was black and white striped.
I have been all over the internet looking for this thing and while I refused to allow it in the truck with me, I would still like to know what it was and whether or not it was dangerous. (yes we let it live)
Is there anyone who can help me with this and tell me where i can find the information on it and what it is?
Thank you
--Dyanna1 09:05, 31 August 2007 (UTC)
- Did you take a picture we can see (but note, I have no idea, but don't know much about bugs, sorry)? --Cody.Pope 09:57, 31 August 2007 (UTC)
- Did it have wings? Was it similar to anything you've seen before? --Ukdan999 13:13, 31 August 2007 (UTC)
- The folks at whatsthatbug.com know their stuff. A lot of potato beetles have black and white stripes, but not large jaws. --Sean 14:52, 31 August 2007 (UTC)
No, unfortunately I did not get to the camera to get a picture of it. It resembled an ant, but it only had its head and body. It was not a "three part body" like any other ant, it was only two parts. No wings.
As I said earlier, I have been all over the net and have found nothing in the ant, beetle, or spider families that even come close. Only its body was black and white striped, its head was orange.
--Dyanna1 17:11, 31 August 2007 (UTC)
- It sounds to me like a type of arachnid called a Solpugid (Order Solfugae). It has a number of common names: sun spider, wind scorpion, and camel spider. It has huge, very powerful jaws that can inflict a very painful bite, but is is not venomous. It usually has a reddish or orange head and a body that is not really striped, but is banded. Try an image search on google and see it that's your critter.--Eriastrum 18:50, 1 September 2007 (UTC)
- Maybe a little late, but there is maybe a small possibility it is an ant. Either a male or a young queen. They often don't look like ants but more like wasps of some sort or another (ants evolved from wasps). They usually have wings, but can loss them pretty easily (the queen breaks off her wings after she has mated and males of some species have their wings removed by workers when the enter a colony to mate). This is just a guess though. --Cody.Pope 12:07, 6 September 2007 (UTC)
Black and white spinner
[edit]Do we have an article on those discs colored with a black and white pattern and when you spin it you see colors? I just got a similar effect while scrolling through a pdf file. Would like more info on the effect - I can't remember how it works. Also, would the two effects be related, I have a clumsy old CRT monitor on this PC and wouldnt be surprised if the effect I just saw was down to some sort of moiré patterning and difference in response times. Capuchin 10:50, 31 August 2007 (UTC)
- We have an article on Benham's top - is that what you mean? DuncanHill 10:58, 31 August 2007 (UTC)
- That's the one I was thinking of, but maybe a version a little less complicated than the picture here. Fechner color seems to describe exactly what I was seeing with the text. Very strange. Capuchin 11:03, 31 August 2007 (UTC)
- I strongly recommend following the link to an interactive version from the Benham's Top page - more optical illusions than you can shake a stick at! DuncanHill 11:23, 31 August 2007 (UTC)
- That's the one I was thinking of! The one with the sets of three lines! Capuchin 11:25, 31 August 2007 (UTC)
- Hmm, on second thought, playing with that interactive one, the black lines appear colored, whereas the effect I was seeing was far more blurry, more of a kind of faint rainbow behind the text. Capuchin 11:28, 31 August 2007 (UTC)
- Wow that is an amazing site. THanks!Phgao 12:22, 31 August 2007 (UTC)
- Hmm, on second thought, playing with that interactive one, the black lines appear colored, whereas the effect I was seeing was far more blurry, more of a kind of faint rainbow behind the text. Capuchin 11:28, 31 August 2007 (UTC)
- That's the one I was thinking of! The one with the sets of three lines! Capuchin 11:25, 31 August 2007 (UTC)
- I strongly recommend following the link to an interactive version from the Benham's Top page - more optical illusions than you can shake a stick at! DuncanHill 11:23, 31 August 2007 (UTC)
- That's the one I was thinking of, but maybe a version a little less complicated than the picture here. Fechner color seems to describe exactly what I was seeing with the text. Very strange. Capuchin 11:03, 31 August 2007 (UTC)
- I believe that the reason is that the red and blue sensors in your eyes are a bit slower than your green sensors. So when white light changes abruptly to black (or vice-versa), your green sensor recognises the change a little ahead of red/blue. So when the pattern moves but your eyes stay still, the red/blue part of the white bars lags a little behind the green part. Since red+blue=magenta, you get magenta down the edges of the strips moving one way and green down the edges of the other. But we are all unique individuals and for some of us the red sensor is a little faster than the blue - so then you get red+green=yellow in one direction and blue in the other. Yet other people don't see the illusion at all. Probably our brains compensate for this effect in normal vision - so it takes some kind of weird-shaped artificial pattern to make the illusion really work well. Anything that flips from black to white quickly ought to exhibit the same effect - so the spinning black/white disks and all of those other effects are showing the same artifact. SteveBaker 14:50, 31 August 2007 (UTC)
An excellent analysis of this effect is "Cohen, Jozef and Gordon, D. A. "The Prevost-Fechner-Benham Subjective Colors." Psychological Bulletin, Volume 46, pages 97-136, 1949. The 1960's black and white TV show "Thriller" hosted by Boris Karloff had an episode in which a woman wearing a dress with vertical black and white stripes was dancing, and when she spun around, the sequence of black and white movement caused all of us viewing it to see red streaks- on the black and white set. Quite unintentional but amazing. If it works on B&W TV it should work on a B&W monitor, but seeing color effects on a color monitor is never going to be very impressive. The disc can be found on the web and in textbooks, and can be printed out in a convenient size, like 8 inches, glued to cardboard, then attached to a machine screw with 2 washers and a bolt(after drilling a hole in the center of the disk). Then simply put the bolt in the chick of a variable speed drill and try spinning it at various speeds forward and backward, to get a variety of effects. Edison 00:57, 2 September 2007 (UTC)
The Membrane Cell for NaOH Production
[edit]Hello! I read that in the Membrane Cell there is a "Semipermeable Plastic Membrane" which seperates the solutions of ions and products. It allows positive Na+ ions to migrate through to react with OH- to form NaOH, yet it prevents the OH- and Cl- ions from passing through. ie. It allows +ve charged ions to pass, yet blocks negative charged ions. I am wondering what material this is, how it works and whether wikipedia has any detailed infomation on it. Thanks! —Preceding unsigned comment added by Phgao (talk • contribs) 11:44, 31 August 2007 (UTC)
- It partially depends on the concentration gradient. You might check out active transport versus passive transport, and things like sodium potassium pumps. --slakr(talk) 11:48, 31 August 2007 (UTC)
- Another one: osmosis, which is basically passive transport/diffusion but with movement of only water. Also, if you're interested in an all-encompassing, practical example, you might check out action potentials, as they're how neurons in your brain communicate. --slakr(talk) 11:52, 31 August 2007 (UTC)
- You know, on third thought, I completely misread what you originally posted, LOL. You were asking what the semi-permeable membrane could be made of. A practical example is dialysis tubing, which is, in essence, a semi-permeable membrane used in real-world kidney dialysis. Sorry 'bout that. --slakr(talk) 12:02, 31 August 2007 (UTC)
- No problem, when I first read what you wrote, I was wondering... So these tubings allow +ve ions to pass while blocking -ve ions? Can you explain why that occurs? —Preceding unsigned comment added by Phgao (talk • contribs) 12:05, 31 August 2007 (UTC)
- You know, on third thought, I completely misread what you originally posted, LOL. You were asking what the semi-permeable membrane could be made of. A practical example is dialysis tubing, which is, in essence, a semi-permeable membrane used in real-world kidney dialysis. Sorry 'bout that. --slakr(talk) 12:02, 31 August 2007 (UTC)
- Another one: osmosis, which is basically passive transport/diffusion but with movement of only water. Also, if you're interested in an all-encompassing, practical example, you might check out action potentials, as they're how neurons in your brain communicate. --slakr(talk) 11:52, 31 August 2007 (UTC)
- Well, it's not really that they're normally specific to the ions-- after all, Na+, Ca2+, and OH-, for example, are extremely small and can easily pass through a cell membrane (this is the basis for diffusion). Alternatively, they can use ion channels. This is most likely too complex for the every day person (and/or biology/chemistry student to have to worry about), though. When someone says that an arbitrary membrane is/isn't permeable to a specific ion, it's usually done in the context of a U-tube problem or something similar, since most biological membranes are always permeable to most small ions-- especially elemental ions (like Na+ and Cl-). As far as I know, the overwhelming majority of the time, the only real way for a membrane to not be permeable to an elemental ion would be if the membrane actually reacted with an ion, thus trapping it in the membrane in some way, but that's probably more complex than what you're dealing with.
- In any case, the main thing you must grasp is the concept of diffusion, as it applies in all circumstances. Say, for example, a membrane happens to be permeable to sodium ions (Na+) but not to starch-- the duodenum of the small intestine is a perfect example. If the concentration of sodium ions on one side of the membrane (say, the blood stream side) is low, and the membrane is permeable to sodium, sodium will pass through the membrane due to the concentration gradient. That is, there are fewer Na+ on the blood side as opposed to the digestive side, so in order to reach chemical equilibrium, Na+ diffuses to the area of lower Na+ concentration. However, regardless of the starch content of the blood stream, since the membrane isn't permeable to starch, starch cannot simply pass through the intestinal wall-- it needs to be broken down into something smaller. That doesn't mean, however, that starch is completely out of the equation.
- Let's instead assume that the membrane were only permeable to water and that everything else was equal. If there's a bunch of starch on one side of the membrane (that is, the starchy side of the membrane has less water in proportion to the all-water side), then water, like sodium, will move across the membrane from the environment of tons of water to the environment with less water-- again, in an attempt to establish equilibrium. This is where terms like hypotonic, hypertonic, and isotonic come in to play. --slakr(talk) 13:40, 31 August 2007 (UTC)
- Thanks for the super info! So it is ion channels which prevent negative ions passing through while allowing positive ions? As these ions would be similar size and the concentration of these would be zero on the other side of the cell, so equilibirum doesnt come into effect here i dont think. Phgao 17:49, 31 August 2007 (UTC)
- Also, I dont mean to say this membrane is specific to these ions, (namely Cl- and Na+), but that since the cell only has Cl-, OH-, Na+, those are the ions in question. Furthermore it is described as some kind of polymer "form of a specially designed plastic sheet that only allows sodium ions to move from one chamber to another i.e. the chloride ions in the anode chamber cannot move into and contaminate the sodium hydrozide produced at the cathode. Furthermore the OH- ions formed at the cathode are prevented from moving to the anode). Phgao 17:52, 31 August 2007 (UTC)
Blueprints
[edit]Is the origin of the term "blueprint" from the fact that photocopiers cannot copy blue colours very well, hence the plans could not be stolen? 82.198.250.8 12:41, 31 August 2007 (UTC)
- This is an encyclopedia. Type blueprint in the search box and click on "Go". -- Kainaw(what?) 12:43, 31 August 2007 (UTC)
- Blueprints predate photocopiers by quite some time, i'm sure :) Capuchin 12:44, 31 August 2007 (UTC)
- Then, later (but still before Xerographic copiers), diazo copiers came into use. While technically producing whiteprints, the output of these copiers was a bluish image on a white background, allowing the continued casual use of the term "blueprint". Ahh, the reek of ammonia!
- I am reminded of a bit in the novel A Canticle for Leibowitz. Civilization has collapsed and there is this monk who has somehow come into possession of a blueprint without knowing what it means. He goes to great effort to copy it with a pen, carefully inking in all the blue areas and leaving the white lines and lettering un-inked, because he assumes that the blue is significant and redrawing it the way we would would not produce an equivalent. --Anonymous, 22:28 UTC, August 31, 2007.
- Blueprints also came in a version with blue lines on a white background, which would have made life easier for the scribe in the excellent book "A Canticle for Liebowitz." Edison 00:52, 2 September 2007 (UTC)
eyesight
[edit]who is the best eye surgoen in leeds —Preceding unsigned comment added by 82.2.218.57 (talk) 13:11, 31 August 2007 (UTC)
- "Best" in this case is a matter of opinion. What are you basing your standards on? -- Kainaw(what?) 14:31, 31 August 2007 (UTC)
- Is Google your friend? We hardly can advise on this matter. Conscious 18:06, 31 August 2007 (UTC)
- Where? --h2g2bob (talk) 00:45, 1 September 2007 (UTC)
- Oh come on. That's like asking where?? when someone asks about something in London. Unless there's some reason to think otherwise, it's safe to assume he means Leeds. -Elmer Clark 21:19, 2 September 2007 (UTC)
- Where? --h2g2bob (talk) 00:45, 1 September 2007 (UTC)
- Is Google your friend? We hardly can advise on this matter. Conscious 18:06, 31 August 2007 (UTC)
Cobalt weight vs Nickel weight
[edit]
Why is Ni, succeeding Co on the periodic table, heavier than the one before it? 81.93.102.185 13:57, 31 August 2007 (UTC)
- The periodic table is ordered by the number of protons in the nucleus. Ni has 28 and Co has 27. However, for the most common isotopes: Ni has only 31 neutrons while Co has 32. Since neutrons are a teeny bit heavier than protons, Co ends up having a slightly higher atomic weight whilst having one less proton. SteveBaker 14:39, 31 August 2007 (UTC)
- Thank you for that speedy reply. Am I to understand that 'most common' means the same as occuring most often in nature, percentagewise? Is there a version of the periodic table which states the most normal isotopes for them? 81.93.102.185 14:46, 31 August 2007 (UTC)
- Probably. But you can see this information on the Wikipedia pages for each element. Go to Nickel and Cobalt and you'll see tables of isotopes for each one. Many isotopes are radioactive - lots of them don't exist at all in nature and have to be manufactured in a nuclear reactor or something. Some of them decay rapidly into the more common isotopes. So, for example, there is Cobalt-56 which has two less neutrons than the naturally occuring Cobalt-58, and there is Nickel-61 which has three more neutrons than Nickel-58. If you happened to pick those two (weird) isotopes instead of the common ones, you'd find that nickel was heavier than cobalt. Since chemical properties are most often related to the number of electrons (which in turn matches the number of protons), it is more useful to have the table of the elements arranged by number of protons than by atomic weight because you get pleasing groupings of elements that behave similarly and predictably from one row to the next. Hence the slightly strange ordering of Nickel and Cobalt. SteveBaker 15:06, 31 August 2007 (UTC)
- (EC) Yes, that's what most common means, and yes, many extended periodic tables will list isotope percentages as part of their data. The excellent online one at www.webelements.com makes it easy to see the percentages (click an element, then "Naturally occurring isotopes" on the left), but it's not all on one page like in some paper ones. That's tough on the web. --Sean 15:10, 31 August 2007 (UTC)
- Thank you for that speedy reply. Am I to understand that 'most common' means the same as occuring most often in nature, percentagewise? Is there a version of the periodic table which states the most normal isotopes for them? 81.93.102.185 14:46, 31 August 2007 (UTC)
- It may be worth noting that Mendeleev had nickel and cobalt backwards for this reason - his arrangement of the elements was based on mass (since the theory of nuclei was still in development). Nimur 16:46, 31 August 2007 (UTC)
wbc
[edit]is it possible to seperate W.B.C. from the blood & inject into the blood of the needy? how can it be done? where the facility available? --121.247.221.158 14:26, 31 August 2007 (UTC)lalitha.
- Yes, it is possible. Type "white blood cell separation" into Google and you'll find many methods. You're asking for non-destructive methods. As for injecting it back, there is an issue with deterioration of the white blood cells. Is there a facility available? I do not believe so. Where's the profit in storing white blood cells for people? -- Kainaw(what?) 14:31, 31 August 2007 (UTC)
- This would not work, actually. White blood cells are created to target completely random antigens. Each person's own body has methods of filtering immature white blood cells of the ones that target "self" antigens, so that only those white blood cells that target foreign antigens make it into maturity. Mature white blood cells harvested from one individual's blood stream would inevitably target normal, human cells in any other individual's body (except an identical twin). The only way to do this properly is to perform a bone marrow transplant, which transfers the stem cells that create white blood cells (among others). Once transplanted, the transplantee's body can still perform the normal function of filtering out self-targetting white blood cells (usually). Someguy1221 14:44, 31 August 2007 (UTC)
The E
[edit]What does E mean in E=mc^2? —Preceding unsigned comment added by 66.213.124.227 (talk) 15:04, 31 August 2007 (UTC)
- Wikipedia has articles on such things, so need to wait for an answer here: just type "E=mc^2" into the search box, press "Go", and all will be revealed. Or click here: E=mc^2. --Sean 15:13, 31 August 2007 (UTC)
- It stands for 'Energy'. In words, E=Mc2 means: Energy equals Mass multiplied by the square of the Speed Of Light. SteveBaker 15:18, 31 August 2007 (UTC)
- Specifically, it's the rest energy, a particular form of the energy of
a particleany object with mass. Other kinds of energy (such as kinetic energy and electric potential energy) have other equations of description. Nimur 16:39, 31 August 2007 (UTC)
- Specifically, it's the rest energy, a particular form of the energy of
- It stands for 'Energy'. In words, E=Mc2 means: Energy equals Mass multiplied by the square of the Speed Of Light. SteveBaker 15:18, 31 August 2007 (UTC)
Burning gas indoors
[edit]Why is it safe to burn natural gas indoors (as with a gas stove or oven), but not to use a gas grill indoors? Doesn't burning natural gas put off carbon monoxide? Thanks. --Sean 17:55, 31 August 2007 (UTC)
- Natural gas is mostly methane. Gas grills use propane. I'm not quite sure if that's why, though. Bart133 (t) (c) 19:11, 31 August 2007 (UTC)
- That is indeed a large part of the reason why. See Propane#Properties_and_reactions. Two things to note:
1) Methane almost always burns "clean" as water and carbon dioxide. Propane can sometimes produce carbon monoxide.
2) A natural gas (methane) leak will shoot gas straight up. If you get lucky, it will wander out of your house very quickly. A liquid petroleum gas (propane) leak will lurk on the floor, so it's harder to get out of the house.
But I should add a third thing -- it's also that gas grills are designed to be used outside, and stoves are designed to be used inside. The technology exists to build indoor propane grills and outdoor methane grills. There's just not a market for them. --M@rēino 19:46, 31 August 2007 (UTC)
- In my previous residence, I had an indoor gas range that used LP (although it could also use natural gas, there was no natural gas supply in my neighborhood). I recall having had to call plumber to do the installation, which partially involved adjusting the incoming gas pressure. So I guess you can use LP inside, but again, it probably has a lot to do with the design of the appliance. --LarryMac | Talk 20:20, 31 August 2007 (UTC)
I moved into a house once with a natural gas furnace that, after a renovation by the previous owner, was in a small room with an ordinary door and no window. A house inspector told me that this was dangerous and should be corrected at once by putting ventilators in the door. (Yes, I did.) Otherwise the natural gas could burn incompletely and it then would indeed produce carbon monoxide. --Anonymous, 23:18 UTC, August 31, 2007.
I have a gas oven and it always builds up this black oily residue on the floor under and adjacent to it. I had always assumed the natural gas had crap in it. Juanita Hodges 00:34, 1 September 2007 (UTC)
- Juanita - please get your oven checked by a qualified gas-fitter. This is not normal behaviour for a gas cooker. DuncanHill 00:37, 1 September 2007 (UTC)
- I think it's fine. It's extremely slow and I've rented the place for 5 years and nothing's happened. So I'm pretty sure it's safe. I just thought somebody might know what the stuff was. Juanita Hodges 00:45, 1 September 2007 (UTC)
- I'd say that's evaporated grease from the food you cooked in there, which then condenses on the floor. In other words, it has nothing to do with the combustion gas. StuRat 01:14, 1 September 2007 (UTC)
- We're not allowed to answer medical questions because readers could be stupid enough to not realise that this ref desk is no substitute for a doctor. I find that very annoying. But in this case I'm inclined to say that a proper diagnosis of Juanita's situation is not possible with the skimpy info provided. People are notoriously unaware of how dangerous a bad gas installation can be. I wouldn't advise to 'go see a doctor' (a 'gas doctor' in this case), but I certainly wouldn't say everything's fine, based on the info at hand. DirkvdM 05:50, 1 September 2007 (UTC)
- Well, regardless of the source, we can say that the greasy spots should be kept clean, since they're possibly flammable and near an ignition source. StuRat 06:15, 1 September 2007 (UTC)
- I believe the answer to the heart of the question is that Natural Gas has a specific Density that varies between .6 and .7 (since it is not all composed of the same molecule) While Propaine on the other hand has a specific density of 1.52; Im sure that you can easily see the dangers of this if there is even a small leak over enough time inside of a confined space. Expecially if there is a basement, etc...--Aaron hart 21:10, 2 September 2007 (UTC)
Young snakes
[edit]How easy is it to distinguish between the young of an adder and of a grass snake? -- 82.26.220.137 19:08, 31 August 2007 (UTC)
- Seriosuly - read about "adder young" and "grass snake young" using wikipedia or a library or search the internet - then compare the differences between the two.
- Before asking a question on the reference desk please do you own research.87.102.87.15 10:43, 1 September 2007 (UTC)
- Please do not WP:BITE persons who ask very reasonable questions on the Ref Desk. Edison 00:48, 2 September 2007 (UTC)
- Then why am I expected to answer it then - why don't you do that,87.102.42.128 08:42, 2 September 2007 (UTC)
- Not everyone spots questions as fast as other people. x42bn6 Talk Mess 09:59, 2 September 2007 (UTC)
- Then why am I expected to answer it then - why don't you do that,87.102.42.128 08:42, 2 September 2007 (UTC)
- Please do not WP:BITE persons who ask very reasonable questions on the Ref Desk. Edison 00:48, 2 September 2007 (UTC)
any thoughs were I can source this —Preceding unsigned comment added by Tobiasatkinson (talk • contribs) 19:13, 31 August 2007 (UTC)
- To clarify -- I'm pretty sure the question is where one would find sources to confirm the info in the riverstone pebble tiles article. (I wish I knew; I'd do it myself.) --M@rēino 19:48, 31 August 2007 (UTC)
- If that is the case the trade descriptions should do in absense of manufacturer information - if the aim is to prove that they exist and are what the article says what they are. (See previous links)83.100.249.228 15:53, 1 September 2007 (UTC)
- I think this was addressed above - Wikipedia:Reference Desk/Science#Riverstone pebble tiles --LarryMac | Talk 20:24, 31 August 2007 (UTC)
In an aqueous solution, why is [H][OH] always equal to a constant?
[edit]I understand that autoionization of water will result in the same [H] and [OH] because as a hydronium ion forms, a hydroxide ion is left behind. But I don't understand what happens if an acid is added. For example, in my chem book, it shows that if .1 M of HCl is added, then there will be 10-12 of [OH]. That's because [H][OH] is always equal to 10-14. Substituting [H] for .1 would yield [OH] to the aforementioned number. I get how to solve the simple equation, but what I need to understand is what actually happens in the molecular level. From my intuition, adding HCl, thus adding protons, will make [H] a lot higher, but I don't know why the [OH] would become lower so that [H][OH] stays constant.128.163.160.121 19:34, 31 August 2007 (UTC)
- Well, you can look at it this way...Whenever you have hydrogen ions and hydroxide ions floating in solution (I'm ignoring that hydronium bit), there's always a probability of the two binding together and becoming water. And, similarly, there's a chance of water spontaneously splitting into those two ions again. When you dump hydrogen ions into an aqueous solution, you greatly increase the chance of a hydrogen ion and hydroxide ion bumping into eachother and forming a water molecule. The chance of a water molecule spontaneously splitting hasn't changed though. Thus, buy dumping hydrogen ions into the solution, you decrease the concentration of hydroxide ions. The familiar equation to us all, Ka=[X+][Y-]/[XY], is what relates all of these probabilities. I forget how to prove this is the case though, I'm sure someone else around here can explain that, or point to a good article. Someguy1221 20:15, 31 August 2007 (UTC)
- To expand on the above, in a water solution you always have the reaction H+ + OH- <-> H2O going on. This is a fast reaction, so it rapidly reaches chemical equilibrium. Due to thermodynamic principles I don't quite fully understand, when a reaction is at equilibrium, you satisfy the equation given above, e.g. Keq = [H+][OH-]/[H2O], where Keq is the equilibrium constant, (and since the reaction is an acid-base reaction, it also happens to be an acid dissociation constant). Since in aqueous solution the concentration of molecular water is practically constant, and Keq is a constant you can move those to the left side of the equation, giving <constant> = [H+][OH-]. Normally, this constant is denoted Kw, and is called the self ionization constant of water. -- 21:27, 31 August 2007 (UTC) —Preceding unsigned comment added by 72.33.121.200 (talk)
- Well, Someguy1221's microkinetic explanation does pretty much explain those "thermodynamic principles". Another way of putting it is that, for H+ + OH- <-> H2O to be in equilibrium, the rates of H2O -> H+ + OH- and H+ + OH- -> H2O must obviously be equal. The first reaction describes the spontaneous breakup of a water molecule, which happens at a constant rate per molecule. Its overall rate therefore depends only on the concentration of water, which, in an aqueous solution, can be treated as a constant. The second reaction, however, involves the collision of a hydroxide ion and a hydrogen ion, an event that happens with a frequency proportional to the respective concentrations of each ion. Thus, when you increase the number of hydrogen ions by adding acid into the solution, that reaction will run faster until the number of hydroxide ions has gone down enough to make the rates equal again. (The actual numerical value of the equilibrium constant Kw, which relates the concentrations needed to achieve this equilibrium, comes from both the rate of spontaneous breakup of water and from the probability of hydroxide and hydrogen ions reacting when they do happen bump into each other.) —Ilmari Karonen (talk) 18:26, 1 September 2007 (UTC)
- The explanations make sense so far, but I don't know why Kw applies to non pure solutions. I thought Kw, the ion product, only applies for pure water. So when HCl or any other substances is added, the solution isn't pure water anymore, thus my confusion as to why Kw applies in the equation. —Preceding unsigned comment added by 128.163.224.199 (talk) 18:59, 2 September 2007 (UTC)
- Well, Someguy1221's microkinetic explanation does pretty much explain those "thermodynamic principles". Another way of putting it is that, for H+ + OH- <-> H2O to be in equilibrium, the rates of H2O -> H+ + OH- and H+ + OH- -> H2O must obviously be equal. The first reaction describes the spontaneous breakup of a water molecule, which happens at a constant rate per molecule. Its overall rate therefore depends only on the concentration of water, which, in an aqueous solution, can be treated as a constant. The second reaction, however, involves the collision of a hydroxide ion and a hydrogen ion, an event that happens with a frequency proportional to the respective concentrations of each ion. Thus, when you increase the number of hydrogen ions by adding acid into the solution, that reaction will run faster until the number of hydroxide ions has gone down enough to make the rates equal again. (The actual numerical value of the equilibrium constant Kw, which relates the concentrations needed to achieve this equilibrium, comes from both the rate of spontaneous breakup of water and from the probability of hydroxide and hydrogen ions reacting when they do happen bump into each other.) —Ilmari Karonen (talk) 18:26, 1 September 2007 (UTC)
- To expand on the above, in a water solution you always have the reaction H+ + OH- <-> H2O going on. This is a fast reaction, so it rapidly reaches chemical equilibrium. Due to thermodynamic principles I don't quite fully understand, when a reaction is at equilibrium, you satisfy the equation given above, e.g. Keq = [H+][OH-]/[H2O], where Keq is the equilibrium constant, (and since the reaction is an acid-base reaction, it also happens to be an acid dissociation constant). Since in aqueous solution the concentration of molecular water is practically constant, and Keq is a constant you can move those to the left side of the equation, giving <constant> = [H+][OH-]. Normally, this constant is denoted Kw, and is called the self ionization constant of water. -- 21:27, 31 August 2007 (UTC) —Preceding unsigned comment added by 72.33.121.200 (talk)
- Fist off H+ is actually H3O+ and [H3O+][OH-]=Kw O.K. now that that is straight, we make the assumption that in an aqueous solution the concentration of water is large compared with the concentration of its ions, thus it is an invarient. This is all you need to be concerned with for now. If you wish to think about the concentrations of a mixture of solutions, whose equations get rather complicated, basically you solve them with a calculator, etc.. But remember the equations are dynamic, for example the chemical ratio formulas on the microscopic level are moving back and forth keeping the ratio. In other words in a complex aqueous solution, no matter what you add to the soulution all the ratios of the ions will remain the same! They stay in eqiuilibrium if you can relate to this you are on your way--Aaron hart 22:25, 2 September 2007 (UTC)
- I forgot to mention with solutions each compound has a Ksp for solubility product constants, there is one for each solution and a K for keeping each compund that ionizes to a constant ratio between the two, these are independent of the other compounds added as far as the ratios go, but it will change the amounts, depending on what you add. The important part is all the ratios of the ions for each chemicals K stays the same regardless of if you have a solution with five compounds, and add a little more of a certain compound the amounts change but all the ratios stay the same.--Aaron hart 22:35, 2 September 2007 (UTC)
Hydrophobic vs. Hydrophilic
[edit]Hydrophilic molecules like water because they're polar. Polar - polar interaction betw. the hydrophilic molecule and water is why they "stick together". But what does it mean when a molecule is hydrophobic? In the wiki page, it says a molecule is h-phobic because it "prefers" non polar molecules. That doesn't sound right to me. Isn't something hydrophobic because it doesn't prefer polar molecules? The wiki page implies that there's an interaction betw non polar molecules. There's no physical attraction between non polar molecules as there are between polar molecules. Am I getting this right?128.163.160.121 19:46, 31 August 2007 (UTC)
- You can have Van der Waals forces between non-polar molecules, but these are weaker than polar-polar interactions. Non-polar molecules can interact with polar ones, but highly polar molecules like water will bind to eachother so strongly as to exclude non-polar molecules except in very low concentrations. Someguy1221 20:09, 31 August 2007 (UTC)
- Right. Each non-polar molecule feels a stronger attraction to the polar molecules than to its own kind, but the polar molecules have such a strong attraction to each other that they form a kind of "clique", and no (or very few) non-polar molecules can break in, even though they'd "like" to. —Keenan Pepper 20:50, 31 August 2007 (UTC)
It's simple -
- Technically a molecule is hydrophobic if the bonding (read 'preference') is stronger with itself than with water - this typically equates to lack of functionality that can hydrogen bond/no charge on the molecule.
- The physical attraction between non polar molecules (see Van der Waals force) is much weaker than that between polar molecules.87.102.87.15 13:14, 1 September 2007 (UTC)
- Effectively you compare the 'preference'/bonding between the molecules you are looking at.87.102.87.15 13:14, 1 September 2007 (UTC)
If you think about it you will see that "prefers non polar molecules" is the same as "doesn't prefer polar molecules" - molecules are either polar or non polar right?83.100.249.228 15:50, 1 September 2007 (UTC)
- They can also be amphipathic. Someguy1221 22:25, 1 September 2007 (UTC)
- Ooooh. I know I'm late to this discussion, but this question hilights a minor but basic misunderstanding about hydrophobic interactions. Hopefully I can help clear that up. In actuality, "hydrophobic interactions" as a descriptor for the force that drives, say, acyl chains on lipids together is kind of a misnomer...the most prominent force that drives fatty acid chains together is actually the entropy of the surrounding aqueous environment. I'll explain:
- Molecular interactions are dictated by changes in a system's Gibbs free energy (ΔG) in which a reaction with negative ΔG will proceed forward
- ΔG is dictated by changes in enthalpy (ΔH is essentially the amount of heat absorbed or released by a reaction; -ΔH is heat released) and entropy (ΔS is essentially the change in a system's order; +ΔS is greater degrees of total freedom); simply, ΔG = ΔH - ΔS
- Pure water has a high degree of entropy as the molecules have many degrees of freedom in movement and organization
- A lipid chain does interact with water, but water interacting with a lipid chain must become significantly more ordered (-ΔS) to orient the polar water molecules with the apolar carbon chains in the most energetically favorable way
- Add more lipid chanis: in water, lipid chains interacting with other lipid chains provide a -ΔH from van der Waals interactions, but have a roughly equal loss of entropy (-ΔS)...
- ...which leaves water: as lipid molecules come together, less water needs to interact with the apolar chains which dramatically increases the entropy of the water, which in turn drops the ΔG of the entire system.
- So, in essence, while lipids will weakly stick together, it's realy more like the water pushing the lipids together that is driving force behind hydrophobic interactions. The same is true for protein folding kinetics as well. — Scientizzle 22:08, 6 September 2007 (UTC)
- Ooooh. I know I'm late to this discussion, but this question hilights a minor but basic misunderstanding about hydrophobic interactions. Hopefully I can help clear that up. In actuality, "hydrophobic interactions" as a descriptor for the force that drives, say, acyl chains on lipids together is kind of a misnomer...the most prominent force that drives fatty acid chains together is actually the entropy of the surrounding aqueous environment. I'll explain:
Escape velocity of the solar system for the sun
[edit]If the sun wanted to leave the solar system, would its required escape velocity be any less than the escape velocity of a deep space probe due to the difference in mass? --frotht 21:42, 31 August 2007 (UTC)
- I'm not sure I understand the question you're asking. I'm guessing you're asking what speed of acceleration/overall speed the sun would require in order to break the gravitional forces that keep the planets etc in orbit? Exxolon 22:01, 31 August 2007 (UTC)
The escape velocity doesn't depend on the mass of the escaping object, but the sun would have a much lower escape velocity anyway because it doesn't have to escape from itself. It only has to escape from the rest of the mass of the solar system, which totals something like 0.2% of the sun's mass. It's not as simple as a ratio of because you also have to factor in the varying distance of the mass from the sun. I suppose for a first approximation we can just take Jupiter, for which I get an escape velocity of 570 m/s, about 70 times smaller than the escape velocity from the solar system from earth orbit.(This is wrong; see Anonymous below for the right answer) -- BenRG 22:03, 31 August 2007 (UTC)
- Would the direction of the sun in relation to the Ecliptic make any difference? i.e. if the sun were to be accelerated directly "up" on a Z-axis in relation to the planets orbiting would that change the velocity required as opposed to be accelerated out on the X-axis "through" the planetary orbits? Exxolon 22:30, 31 August 2007 (UTC)
- It would make a slight difference, in that the distance from most of the non-Sun mass of the solar system would increase more quickly going perpendicular to the plane in which most objects orbit. While the gravitational attraction as the Sun approached those objects would help speed it up, it's faster speed than after it passed those objects would mean the drag would have more time to act than the attraction, sort of a reverse slingshot effect. StuRat 22:40, 31 August 2007 (UTC)
The statement that "the escape velocity doesn't depend on the mass of the escaping object" only works where the primary is massive enough that you can treat it as stationary. Let's consider the solar system as simplified all the way to "Jupiter plus debris". What speed would have to be imparted to Jupiter (mass for it to escape from the Sun (mass )? What speed would have to be imparted to the Sun for it to escape from Jupiter? You can't just apply the formula and say that it's in the first case and in the second, giving two grossly different answers; we're talking about the exact same situation viewed from two different frames of reference. Either the bodies are moving fast enough with respect to each other than they never fall back together, or they aren't.
The correct frame of reference is, I believe, the one where the center of mass of the Sun and Jupiter is stationary — which is almost the same as the one where the Sun is stationary. This means that is the correct answer for either body to escape
from the other. In SI units and rounding to 2 significant digits, Wikipedia (at Gravitational constant, Sun, and Jupiter) gives ,
, and (based on the semi-major axis). From which I compute that the correct escape velocity is about 13,000 m/s or 29,000 mph. The other planets would add to that, but not a whole lot due to their greater distance and/or much, much lower mass, except for Saturn.
The direction makes no difference, so long as it isn't one that would cause a collision. It's purely a matter of kinetic energy. Escape velocity, as it says in the article, would be better referred to as "escape speed".
--Anonymous, waiting now for 00:00 UTC, September 1, 2007.
- Yes, you're quite right. Sorry. -- BenRG 00:44, 1 September 2007 (UTC)
- So was I right? The sun doesn't have to escape from its own mass so its escape velocity is much lower than a probe's, right? --frotht 02:20, 1 September 2007 (UTC)
- Wrong -- the situation is symmetrical. You can think of it this way. The Sun doesn't have to "escape from its own mass", but it has to go fast enough to keep its own gravity from dragging everything else after it (which wouldn't be escaping).
- But I made a major error too. I said that the other planets wouldn't have much effect because of their relatively small mass, but as I've explained, it's the Sun's mass and the distance that matter. In order for the Sun to "escape" from the entire solar system, it has to escape from the closest body to it, otherwise that body will be dragged along with it. So that's Mercury, whose distance from the Sun varies from to m.
- Which means that the relevant escape velocity is anywhere from 44,000 m/s (98,000 mph) to 54,000 m/s (120,000 mph), depending on where in its orbit Mercury is at the time that the Sun makes its giant leap for freedom.
- --Anonymous, 04:17 UTC, September 1, 2007.
- Is there a rate of acceleration involved here? Or does this calculation assume that the sun jumps from 0 m/s relative to mercury to the 44,000m/s / 54,0000 m/s you've quoted. In laymans terms, how hard does the sun have to accerlerate to snap the "gravitational elastic". If it takes 1 day to reach to 44/54k figure would that be fast enough? Exxolon 16:41, 1 September 2007 (UTC)
- Correct me if I'm wrong anonymous, but the only condition that must be met is that the relative velocity between the sun and mercury has to be ~50km/s. Intuition tells me that basically any acceleration at all will eventually build up that difference, even though "gravity is trying to keep up" by pulling mercury behind it. --frotht 18:00, 1 September 2007 (UTC)
- Is there a rate of acceleration involved here? Or does this calculation assume that the sun jumps from 0 m/s relative to mercury to the 44,000m/s / 54,0000 m/s you've quoted. In laymans terms, how hard does the sun have to accerlerate to snap the "gravitational elastic". If it takes 1 day to reach to 44/54k figure would that be fast enough? Exxolon 16:41, 1 September 2007 (UTC)
- --Anonymous, 04:17 UTC, September 1, 2007.
- The formula for escape velocity applies to a direct jump from zero to full speed. If prolonged acceleration is allowed, there is no requirement for any particular speed. As the acceleration continues, the distance will keep increasing, changing the R term in the formula and thus reducing the escape velocity. At some time the increasing actual velocity will overtake the reduced escape velocity and there you are. However, the energy required to accelerate for enough time to achieve escape at low speed is much greater than the energy required to accelerate rapidly to the usual escape velocity. (Oddly enough, this point came up in a comedy movie once: The Mouse on the Moon, which is not based on any sort of serious physics. They were talking about going to the Moon rather than escaping Earth altogether, but the issue is the same. Professor Kokintz in the movie says something like "They have to go fast because they are using rockets. With my device providing unlimited power, we can take our time getting to the Moon." Which is right.) --Anonymous, 11:46 UTC, September 2, 2007.
- If we think of a simple two-body problem - a rocket and the sun, say - then the answer is very easy. Does the rocket need more velocity than the sun in order to ultimately put an arbitarily large distance between them? The answer has to be "no" because relativity says that the laws of physics are the same no matter your frame of reference. Whether the rocket considers itself stationary and the sun is moving away at escape velocity - or whether it is moving at escape velocity and the sun is stationary - it's the same thing. But if you try to turn that argument into something about the sun escaping the solar system - then you get into trouble because all of the planets, moons, rocks, asteroids (and Pluto) that make up the solar system already have a lot of relative motion. If the sun started moving off, then some of those objects are going to be heading in the same direction - others in the opposite direction. To escape from all of them requires the sun to have sufficient velocity to escape from all of them. That depends a lot on which direction the sun is moving in - and on complicated interactions between the gravitation of the planets themselves. It's a really complicated (and ultimately meaningless) question. 66.137.234.217 19:43, 1 September 2007 (UTC)
Is my Sun Conure in pain??
[edit]She's going through a major moult at the moment and seems to be really irritable. She's scratching, beating her raggedy wings to get the loose feathers in order and fidgeting all the time. She also gets very angry if I try to stroke her and accidentally touch her new pin feathers. Will she be in actual pain from all this? I've seem how deep the roots on some feathers are and the new ones coming through her skin are sharp. --62.136.214.30 22:48, 31 August 2007 (UTC)
- We cannot give veterinary advice on Wikipedia - please consult your usual vet for advice. I do hope that your pet is back to her normal self soon. DuncanHill 23:24, 31 August 2007 (UTC)
- Perhaps more itchy than pain, just like ingrown hair in people. StuRat 01:08, 1 September 2007 (UTC)
- If you are at all concerned about your bird, take her to the veterinarian, even if only to put your mind at rest. My budgies seem to suffer a lot during the biannual 'big moults'. If I accidentally stroke a blood feather (i.e. a growing pin feather that still has a blood supply), they flinch and squeak (then bite me) - which does lead me to believe that there is some level of discomfort there. --Kurt Shaped Box 13:59, 1 September 2007 (UTC)
The gloowwww, the wonderful glowwww!
[edit]Here's a stumpter for you (I hope), I was fiddling around with the settings on my camera. Hue set to 0, saturation to full, gamma middle, white balance full, contrast full. Everything in the picture, including me, went a rich purple, except my eyes which glowed bright green! On close inspection, it was my contact lenses!! Without a doubt, I can see that that the green disc encompases my iris and goes slightly beyond just like my contacts, and i can move them around. Why do my contacts come up green when they dont even show on the camera normally? SGGH speak! 23:35, 31 August 2007 (UTC)
- come on guys, I look like vincent price with this goatee.... SGGH speak! 23:58, 31 August 2007 (UTC)
- I could tell you, but I'd have to run a few spectrophotometric tests on your lenses. — Kieff | Talk 00:49, 1 September 2007 (UTC)
- Flash on? Flash off? --Reuben 00:50, 1 September 2007 (UTC)
- I'm thinking your camera has some type of automatic red-eye adjustment, whereby it adds green/reduces red to what it determines to be your eyes, in order to compensate for this. If you can find such a setting, turn it off and see if the green eyes go away. StuRat 01:02, 1 September 2007 (UTC)
- Did you try it with your contacts out? —Keenan Pepper 01:48, 1 September 2007 (UTC)
Can you give us a picture of something (probably not yourself for privacy reasons but a chair maybe and then contacts inside their case) to analyze better with the unusual setting you did? Juanita Hodges 14:54, 1 September 2007 (UTC)


- Over-saturation tends to pull more or less random colours from greyish areas. For example, these two pictures (original from today's front-page). The smallest hint of colour in the gray parts of the image is enough to throw the math off into generating wild colours that are essentially random. I can explain the math behind it if you care...but it's not very interesting. The answer is "don't do that"! SteveBaker 16:07, 1 September 2007 (UTC)
- That's a great example, Steve. Our reader might also find Saturation, Hue, and HSV color space useful. TenOfAllTrades(talk) 17:40, 1 September 2007 (UTC)
- Was a webcamera, so had no flash or red-eye issues. I suspect the answer involving those nice mountain shots is the best guess. Cheers guys. SGGH speak! 21:44, 1 September 2007 (UTC)
- That's a great example, Steve. Our reader might also find Saturation, Hue, and HSV color space useful. TenOfAllTrades(talk) 17:40, 1 September 2007 (UTC)
- With a webcam the effect would take effect (...) sooner because overall there is less basic colour info to go by (as is the case in grey areas). But I don't think the effect is random - what little colour info there is is grossly exaggerated for lack of anything else to go by. DirkvdM 09:14, 2 September 2007 (UTC)
- I stand by what I said - the colour you get isn't truly random (I explicitly said "essentially random") - but a one bit difference from an 8-bit RGB value of (128,128,128) (mid grey) to (128,127,128) (mid grey but very, very slightly pinkish) is enough to cause the magenta in those mountain tops of the photo on the right, above. The least-significant bit of the value the camera captures in it's CCD is 'essentially' random - it's just not 100% accurate - nothing in nature ever is. So with enough of a saturation boost, the hue of the colours you get out are indeed essentially random because super-saturation magnifies small errors in measured brightness into violent colour changes. SteveBaker 16:15, 2 September 2007 (UTC)
- With a webcam the effect would take effect (...) sooner because overall there is less basic colour info to go by (as is the case in grey areas). But I don't think the effect is random - what little colour info there is is grossly exaggerated for lack of anything else to go by. DirkvdM 09:14, 2 September 2007 (UTC)
- Our disagreeing is essentially a difference in interpretation of the word 'essential', but you're the native English speaker, so I'll yield (although not before pointing out you misspelled 'its CCD' :) ). But back to the point itself. If a colour going one way or another depends on one lsb, then 'another quantum fluctuation' (I probably use the wrong words here) may have caused another value for that bit and then it would have been truly random effect. Still not sure what you mean by 'essentially', though. And while we're talking language, you're from Texas but use EE spelling - how come? DirkvdM 18:27, 2 September 2007 (UTC)
- I think SteveBaker is correct, but he's being a stickler for the definition of random. Since it's a photovoltaic process, the bits are subject to noise, etc, etc, etc. Long integration times should yield the true value, which is due to the deterministic details of the source of the photons. This point, I think, is tangential to the original poster's question. Nimur 18:52, 2 September 2007 (UTC)


















