Valspodar
Appearance
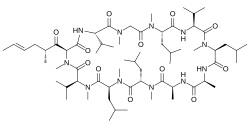
| |
| Names | |
|---|---|
| IUPAC name
(3S,6S,9S,12R,15S,18S,21S,24S,30S,33S)-6,9,18,24-Tetraisobutyl-3,21,30-triisopropyl-1,4,7,10,12,15,19,25,28-nonamethyl-33-[(2R,4E)-2-methyl-4-hexenoyl]-1,4,7,10,13,16,19,22,25,28,31-undecaazacyclotritriacontane-2,5,8,11,14,17,20,23,26,29,32-undecone
| |
| Other names
PSC833; PSC-833
| |
| Identifiers | |
3D model (JSmol)
|
|
| ChemSpider | |
PubChem CID
|
|
| UNII | |
CompTox Dashboard (EPA)
|
|
| |
| |
| Properties | |
| C63H111N11O12 | |
| Molar mass | 1214.646 g·mol−1 |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
| |
Valspodar (PSC833) is an experimental cancer treatment and chemosensitizer.[1] It is a derivative of ciclosporin D (cyclosporin D).
Its primary use is as an inhibitor of the efflux transporter P-glycoprotein. Previous studies in animal models have found it to be effective at preventing cancer cell resistance to chemotherapeutics, but these findings did not translate to clinical success.[2]
Adverse effects
[edit]Valspodar can cause nerve damage.[1]
References
[edit]- ^ a b Wilkes, Gail; Ades, Terri B. (2004). Consumers Guide to Cancer Drugs. Jones & Bartlett Learning. p. 226. ISBN 9780763722548. Retrieved 29 May 2013.
- ^ Tao, Jian'guo; Sotomayor, Eduardo. (2012). Hematologic Cancers: From Molecular Pathobiology to Targeted Therapeutics. Springer. p. 335. ISBN 9789400750289.
