User talk:Nandita93
Appearance
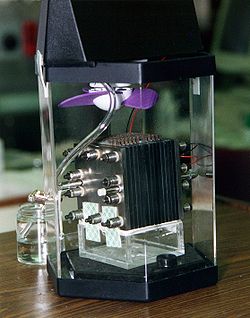
A fuel cell is an electrochemical cell that converts chemical energy from a fuel into electric energy. Electricity is generated from the reaction between a fuel supply and an oxidizing agent. The reactants flow into the cell, and the reaction products flow out of it, while the electrolyte remains within it. Fuel cells can operate continuously as long as the necessary reactant and oxidant flows are maintained.
Start a discussion with Nandita93
Talk pages are where people discuss how to make content on Wikipedia the best that it can be. Start a new discussion to connect and collaborate with Nandita93. What you say here will be public for others to see.

