User:Wppreen/Chemical synthesis
| This is the sandbox page where you will draft your initial Wikipedia contribution.
If you're starting a new article, you can develop it here until it's ready to go live. If you're working on improvements to an existing article, copy only one section at a time of the article to this sandbox to work on, and be sure to use an edit summary linking to the article you copied from. Do not copy over the entire article. You can find additional instructions here. Remember to save your work regularly using the "Publish page" button. (It just means 'save'; it will still be in the sandbox.) You can add bold formatting to your additions to differentiate them from existing content. |
Article Draft
[edit]Lead
[edit]Chemical synthesis (chemical combination) is the artificial execution of chemical reactions to obtain one or several products. This occurs by physical and chemical manipulations usually involving one or more reactions. In modern laboratory uses, the process is reproducible and reliable.
A chemical synthesis involves one or more compounds (known as reagents or reactants) that will experience a transformation under certain conditions. Various reaction types can be applied to formulate a desired product. This requires mixing the compounds in a reaction vessel, such as a chemical reactor or a simple round-bottom flask. Many reactions require some form of processing ("work-up") or purification procedure to isolate the final product.
The amount produced by chemical synthesis is known as the reaction yield. Typically, yields are expressed as a mass in grams (in a laboratory setting) or as a percentage of the total theoretical quantity that could be produced based on the limiting reagent. A side reaction is an unwanted chemical reaction that can reduce the desired yield.
The history of chemical synthesis began with Friedrich Wöhler’s 1828 synthesis of urea from ammonium cyanate, challenging the belief that organic compounds required a “vital force.” [1] This breakthrough demonstrated that organic molecules could be artificially created. In 1856, William Henry Perkin accidentally produced mauveine, the first synthetic dye, during an attempt to synthesize quinine. This discovery spurred the development of synthetic dyes and industrial chemical processes.[1] The word synthesis was used first in a chemical context by the chemist Hermann Kolbe.
Green chemistry focuses on making chemical synthesis more sustainable by reducing waste and improving reaction efficiency.[2] There are many real-world appliications of chemical synthesis.
Article body
[edit]Strategies
[edit]Chemical synthesis employs various strategies to achieve efficient, precise, and molecular transformations that are more complex than simply converting a reactant A to a reaction product B directly. These strategies can be grouped into approaches for managing reaction sequences.
Reaction Sequences:
Multistep synthesis involves sequential chemical reactions, each requiring its own work-up to isolate intermediates before proceeding to the next stage.[3] For example, the synthesis of paracetamol typically requires three separate reactions. Divergent synthesis starts with a common intermediate, which branches into multiple final products through distinct reaction pathways. Convergent synthesis synthesis involves the combination of multiple intermediates synthesized independently to create a complex final product. One-pot synthesis involves multiple reactions in the same vessel, allowing sequential transformations without intermediate isolation, reducing material loss, time, and the need for additional purification. Cascade reactions, a specific type of one-pot synthesis, streamline the process further by enabling consecutive transformations within a single reactant, minimizing resource consumption
Catalytic Strategies:
Catalysts play a vital role in chemical synthesis by accelerating reactions and enabling specific transformations. Photoredox catalysis provides enhanced control over reaction conditions by regulating the activation of small molecules and the oxidation state of metal catalysts, with applications in both organic and inorganic chemistry. Biocatalysis uses enzymes as catalysts to speed up chemical reactions with high specificity under mild conditions.
Reactivity Control:
Chemoselectivity ensures that a specific functional group in a molecule reacts while others remain unaffected. Protecting groups temporarily mask reactive sites to enable selective reactions, they are removed later without damaging the product. Kinetic control prioritizes reaction pathways that form products quickly, often yielding less stable compounds. In contrast, thermodynamic control favors the formation of the most stable products, though the process may require more time or energy.
Advanced Planning and Techniques:
Retrosynthetic analysis is a strategy used to plan complex syntheses by breaking down the target molecule into simpler precursors, guiding chemists toward efficient synthetic pathways. Flow chemistry is a continuous reaction method where reactants are pumped through a reactor, allowing precise control over reaction conditions and scalability. This approach has been employed in the large-scale production of pharmaceuticals such as Tamoxifen.[4]
Organic Synthesis
[edit]Organic synthesis is a special type of chemical synthesis dealing with the synthesis of organic compounds. For the total synthesis of a complex product, multiple procedures in sequence may be required to synthesize the product of interest, needing a lot of time. A purely synthetic chemical synthesis begins with basic lab compounds, while a semisynthetic process starts with natural products from plants or animals and then modifies them into new compounds.
Green Chemistry
[edit]Chemical synthesis using green chemistry promotes the design of new synthetic methods and apparatus that simplify operations and seeks environmentally benign solvents. Key principles include atom economy, which aims to incorporate all reactant atoms into the final product, and the reduction of waste and inefficiencies in chemical processes. Innovations in green chemistry, contribute to more sustainable and efficient chemical synthesis, reducing the environmental and health impacts of traditional methods.[2]


Applications
[edit]Chemical synthesis plays a crucial role across various industries, enabling the development of materials, medicines, and technologies with significant real-world impacts.
Catalysis: The development of catalysts is vital for numerous industrial processes, including petroleum refining, petrochemical production, and pollution control. Catalysts synthesized through chemical processes enhance the efficiency and sustainability of these operations.[6]
Medicine: Organic synthesis plays a vital role in drug discovery, allowing chemists to develop and optimize new drugs by modifying organic molecules.[6] Additionally, the synthesis of metal complexes for medical imaging and cancer treatments is a key application of chemical synthesis, enabling advanced diagnostic and therapeutic techniques.[7]
Biopharmaceuticals: Chemical synthesis is critical in the production of biopharmaceuticals, including monoclonal antibodies and other biologics. Chemical synthesis enables the creation and modification of organic and biologically sourced compounds used in these treatments. Advanced techniques, such as DNA recombinant technology and cell fusion, rely on chemical synthesis to produce biologics tailored for specific diseases, ensuring they work effectively and target diseases precisely.[8]
References
[edit]- ^ a b Schwarcz, Joe (October 22, 2024). "The Beginnings of Chemical Synthesis". McGill.
{{cite web}}: CS1 maint: url-status (link) - ^ a b Li, Chao-Jun; Trost, Barry M (September 9, 2008). "Green chemistry for chemical synthesis". Proceedings of the National Academy of Sciences of the United States of America. 105 (35): 13197–13202.
- ^ "10.10: An Introduction to Multiple Step Synthesis". LibreTextsChemistry.
- ^ "Flow chemistry". Vapourtec. Retrieved 2024-12-01.
- ^ Xu, Z.; Shi, Z.; Jiang, L. (2011-01-01), Moo-Young, Murray (ed.), "3.18 - Acetic and Propionic Acids", Comprehensive Biotechnology (Second Edition), Burlington: Academic Press, pp. 189–199, doi:10.1016/b978-0-08-088504-9.00162-8, ISBN 978-0-08-088504-9, retrieved 2024-12-01
- ^ a b "APPLICATIONS OF ORGANIC CHEMISTRY IN ENGINEERING AND BIOTECHNOLOGY: AN OVERVIEW". Lead & Mentor.
- ^ "Inorganic Synthesis". Socratica.
- ^ "Think : Thermal : Part Two : Uses and Benefits to the biopharmaceutical industry". Thermal Product Solutions. February 10, 2020.
{{cite web}}: CS1 maint: url-status (link)
RH + SL's part:
[edit]**our references start from 7-20
Roy:
Inorganic synthesis
[edit]Inorganic synthesis and organometallic synthesis are used to prepare compounds with significant non-organic content. An illustrative example is the preparation of the anti-cancer drug cisplatin from potassium tetrachloroplatinate.[1]
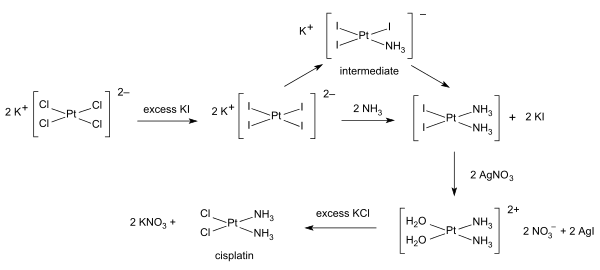
Inorganic Steps
[edit]Inorganic synthesis can be incredibly complex, and involve several intermediates and elementary steps. The steps outlined below are a few of the types of steps that can occur during an inorganic synthesis.
Oxidative Additiion
[edit]Oxidative Addition involves a metal center and a molecule with at least two atoms; the atoms can be of the same species, but do not necessarily have to be. The bond between the two atoms will break, and 2 new bonds will form between those atoms and the metal center. [2]

This is reffered to as "oxidative" because atoms A and B oxidize the metal; that is, the oxidation state of the metal is +2 relative to the oxidation state of the metal before the oxidative addition took place.[2]
Reductive Elimination
[edit]Reductive Elimination is the reverse reaction of Oxidative Addition. It invovles two atoms, A and B, bonded to a metal center. Reductive elimination will see atoms A and B form a bond with each other while both losing their bonds with the metal center.[3]
This is reffered to as "Reductive" because this reaction reduces the metla center; that is, the metal center's oxidation state will be 2 lower than it was before the reaction took place. [4]
Ligand Substitution
[edit]Ligand Substitution occurs when a chemical species attached the a metal center is replaced with a different one.
Associative Substitution
[edit]
Associative Substitution sees an incoming ligand Y coordinate to the metal center as the first step. Only after the association is complete, will the leaving ligand X leave the metal center, completing the substution process.[5]
This mechanism tends to occur with complexes that are unsaturated in both ligands and electrons; that is, ligands with <6 ligands and <18 valence electrons.[6]
The first step is generally rate determining, meaning that the reaction rate is second order; the reaction speed dependsd on both the concentration of the metal center and the concentration of the [Y] species.[7]
Dissociative Substitution
[edit]Dissociative substution sees the outgoing ligand X leave the metal center before the incoming lingad Y coordinates with the metal center. Only after X completely leaves does the new ligand Y coordinate to the metal center.
This mechanism tends to occur with ligands that are fully staurated; that is, complexes with 6 ligands and 18 valence electrons.
The rate determing step tends to be the dissociation step. Thus, the rate tends to be first order; the reaction rate depends on the concentration of the metal center, and is independant of both the concentration and identity of ligand Y.
Susan:
Organic Synthesis
[edit]SN1 Reaction:
[edit]
SN1 is a class of nucleophilic substitution reactions where it involves 2 steps between a central carbon and leaving group. The first step is a slow, rate determining step where the bond between the central atom and leaving group breaks forming a carbocation.[8] The second step is faster, as it involves a nucleophile attacking the empty carbocation.
It removes the leaving group in the molecule and forms a new C-Nu bond. SN1 reactions are best reacted with secondary or tertiary alkyl halides, with tertiary alkyl halides being the most optimal molecule to react with.[8]
SN2 Reaction:
[edit]
Similar to SN1, SN2 is also a class of nucleophilic substitution reactions where it involves 2 steps between a central carbon and leaving group. SN2 reactions are concerted, meaning they are a one step process.[9]
It is a process where the nucleophile attacks an electrophilic carbon, and the bond of leaving group and carbon will be broken at the same time. A set of lone pair electrons from the nucleophile attacks the electrophilic carbon of the alkyl halide to form a sp2 transition state, then forms a new C-Nu bond. SN2 reactions are best reacted with primary or secondary alkyl halides, with primary alkyl halides being the most optimal molecule to react with.[9]
Important Applications in Organic Synthesis:
[edit]Suzuki-Miyaura Cross-Coupling:
[edit]Suzuki-Miyaura Cross-Coupling is a metal-catalyzed reaction involving a palladium catalyst to cross-couple a boronic acid to an organohalide.[10][11] The reaction is used to create new carbon-carbon bonds to make conjugated systems of compounds such as alkenes, styrenes and biaryl compounds.[12] Suzuki coupling involves 3 steps: oxidative addition, transmetallation and reductive elimination.

There are important applications in Suzuki-Miyaura Cross Coupling, as their advantages are that they can react in mild reaction conditions and their starting materials are easy to obtain. They are also known for their low toxicity, as boronic acids are easy to handle and obtain in the market. This reaction is commonly used for synthesizing biaryls, which are important compounds in industrial chemistry and pharmaceuticals.[13] Medical fields utilize biaryls as a key step producing bio-active molecules for medicines. They are privileged structures that have a high affinity and are capable of binding to multiple receptors for biological activity.[14] Other applications include producing polymers for electronic chip insulation and using compounds for organic light emitting diodes. Furthermore, Suzuki-Miyaura Cross-Coupling has important applications for polymer synthesis.
Polymer Synthesis:
[edit]
Polymers are long-chain repeating molecules that are created from monomers, which join together by covalent bonding to form polymer structures.[15] Polymer synthesis has important applications in materials chemistry and medical fields, as they are essential for creating nano materials in medicine which helps diagnose, treat, and prevents certain types of diseases.[16] There are two groups of polymer synthesis reactions: condensation and addition polymerization.

In condensation polymerization, it involves the elimination of small molecules (often water) to form polymers. It happens between two different bi-functional or tri-functional monomers. In addition polymerization, polymer growth requires an initiator that produces the initiator species with reactive centres, such as a free radical, anion, cation, or organometallic complex.
References
[edit]- ^ Alderden, Rebecca A.; Hall, Matthew D.; Hambley, Trevor W. (1 May 2006). "The Discovery and Development of Cisplatin". J. Chem. Educ. 83 (5): 728. Bibcode:2006JChEd..83..728A. doi:10.1021/ed083p728.
- ^ a b Housecroft, Catherine E.; Sharpe, Alan G. (2018). Inorganic chemistry (Fifth edition ed.). Harlow, England London New York Boston San Francisco Toronto Sydney: Pearson. ISBN 978-1-292-13414-7.
{{cite book}}:|edition=has extra text (help) - ^ "Reductive elimination", Wikipedia, 2023-02-12, retrieved 2024-11-05
- ^ "Reductive elimination", Wikipedia, 2023-02-12, retrieved 2024-11-05
- ^ "Associative substitution", Wikipedia, 2022-03-08, retrieved 2024-11-05
- ^ "Associative substitution", Wikipedia, 2022-03-08, retrieved 2024-11-05
- ^ "Associative substitution", Wikipedia, 2022-03-08, retrieved 2024-11-05
- ^ a b Ashenhurst, James (2012-07-13). "The SN1 Mechanism". Master Organic Chemistry. Retrieved 2024-11-03.
- ^ a b "11.2: The SN2 Reaction". Chemistry LibreTexts. 2015-05-03. Retrieved 2024-11-03.
- ^ "Suzuki reaction", Wikipedia, 2024-09-26, retrieved 2024-11-03
- ^ Miyaura, Norio; Yamada, Kinji; Suzuki, Akira (1979-01-01). "A new stereospecific cross-coupling by the palladium-catalyzed reaction of 1-alkenylboranes with 1-alkenyl or 1-alkynyl halides". Tetrahedron Letters. 20 (36): 3437–3440. doi:10.1016/S0040-4039(01)95429-2. ISSN 0040-4039.
- ^ Chemler, Sherry R.; Trauner, Dirk; Danishefsky, Samuel J. (2001-12-17). "The B-Alkyl Suzuki-Miyaura Cross-Coupling Reaction: Development, Mechanistic Study, and Applications in Natural Product Synthesis A list of abbreviations can be found at the end of the article". Angewandte Chemie (International Ed. in English). 40 (24): 4544–4568. doi:10.1002/1521-3773(20011217)40:24<4544::aid-anie4544>3.0.co;2-n. ISSN 1521-3773. PMID 12404358.
- ^ "Suzuki-Miyaura Cross-Coupling Reaction". News-Medical. 2018-01-12. Retrieved 2024-11-03.
- ^ "Suzuki-Miyaura Cross-Coupling Reaction". News-Medical. 2018-01-12. Retrieved 2024-11-03.
- ^ "Polymer Synthesis Techniques". Sigma Aldrich. Retrieved November 28, 2024.
- ^ Zhu, Yuhan (2022-12-30). "New Technologies in Polymer Synthesis and Applications of Polymers". Highlights in Science, Engineering and Technology. 26: 455–460. doi:10.54097/hset.v26i.4026. ISSN 2791-0210.
