User:Wickey-nl/pb

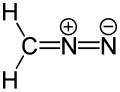
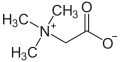
In chemistry, a dipolar compound or simply dipolar is an electrically neutral molecule carrying a positive and a negative charge in at least one canonical description. In most dipolar compounds the charges are delocalized. [1] Dipolar compounds can be represented by a resonance structure. Contributing structures containing charged atoms are denoted as zwitterions. [2] [3] [4] [5] Some dipolar compounds can have an uncharged canonical form.
Types of dipolar compounds
[edit]- 1,2-dipolar compounds have the opposite charges on adjacent atoms.
- 1,3-dipolar compounds have the charges separated over three atoms.[1]
- Betaines are zwitterionic compounds derived from amino acids.[6]
Examples
[edit]See also
[edit]References
[edit]- ^ a b IUPAC Gold Book dipolar compounds
- ^ Braida et al.: A clear correlation between the diradical character of 1,3-dipoles and their reactivity toward ethylene or acetylene.; J. Am. Chem. Soc.; 2010 Jun 9;132(22):7631-7
- ^ Hartmann and Heuschmann: Isolation of a Zwitterion in a Diels–Alder Reaction with Inverse Electron Demand; Angewandte Chemie; september 1989; Volume 28, Issue 9, pages 1267–1268
- ^ Machiguchi et al. Exclusive Formation of α-Methyleneoxetanes ...; J. Am. Chem. Soc., 2003, 125 (47), pp 14446–14448; doi:10.1021/ja030191g
- ^ Rolf Huisgen (IUPAC): Cycloaddition mechanism and the solvent dependence of rate; Pure & Appl. Chem.; 1980, Vol.52, pp.2283—2302.
- ^ IUPAC Gold Book betaines
^^^^^^^^^^^^^^^^^^^^^^^^^^^^^^^^^^^^^^^^^^^^^^
The intense color of PB is caused by the combination of two different oxidation states of the same element in the same complex.
The intense blue color of Prussian blue is associated with the energy of the transfer of electrons from Fe(II) to Fe(III). Many such mixed-valence compounds absorb certain wavelengths of visible light. In this case, orange-red light around 680 nanometers in wavelength is absorbed, and the transmitted light appears blue as a result.
Soluble PB:
FeIII atoms are surrounded by an octahedral arrangement of nitrogen atoms; FeII atoms are surrounded by an octahedral arrangement of carbon atoms.
http://pubs.acs.org/doi/abs/10.1021/ic50091a012 Calorimetric study of Prussian blue;
DOI: 10.1021/ic50091a012
absorption spectrum PB = 700 nm
peak ferricyanide = 420 nm
FeIII +ferricyanide → yellowish brown ferric ferricyanide.
http://pubs.acs.org/doi/abs/10.1021/ac00278a041 Photoacoustic spectra of prussian blue ; DOI: 10.1021/ac00278a041
References
[edit]FeIII chloride + potassium ferricyanide → Berlin green
or CL + potassium ferrocyanide