User:Wang226/Spheroidite
| Spheroidite | |
|---|---|
| Composition | α-ferrite, banite, cementite |
| Brinell hardness | 147* |
| Ductility (%RA) | 75* |
| |
Spheroidite is a microstructure found in steel alloys consisting of sphere-like cementite particles within an α-ferrite matrix. It is produced by an appropriate elevated-temperature heat treatment of pearlite, bainite, or martensite[1]. The process of spheroidization can be further explained and described by Rayleigh's perturbation theory and fault migration theory. Spheroidite is relative soft and tough, because the structural damage caused by a crack is contained by minimizing the brittle cementite particles and maximizing the ductile ferrite matrix. The purpose of spheroidization is to increase the workability of high carbon steel, as spheroidite is known as the most ductile form of steel. However,due to high ductility and energy-consuming production process, spheroidite is relatively insignificant in engineering application.
Production
[edit]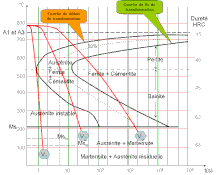
Spheroidite can be produced by heating a pearlitic or bainitic microstructure to and leaving at a temperature below the eutectoid for a sufficiently long period of time—for example, at about 700°C (1300°F) for between 18 and 24 hours. Instead of the alternating ferrite and cementite lamellae (pearlite) or the microstructure observed for bainite, the Fe3C phase would transform into sphere-like particles embedded in a continuous α-phase matrix. This transformation is driven by additional carbon diffusion with no change in the compositions or relative amount of ferrite and cementite phase. The driving force for this transformation is the reduction in α-Fe3C phase boundary area. The kinetics of spheroidite formation are not included on isothermal transformation[2].
Medium-carbon and high-carbon steels with microstructure containing even coarse pearlite may still be too hard to conveniently machine or plastically deform. These steels, and in fact any steel, may be heat-treated or annealed to develop the spheroidite structure. Spheroidized steels have a maximum softness and ductility and are easily machined or deformed. The spheroidizing heat treatment, during which there is a coalescence of the Fe3C to form the spheroid particles, can take place by several methods, as follows: [3]
- Heating the alloy to a temperature just below the eutectoid in the α + Fe3C system. If the precursor microstructure contains pearlite, spheroidizing times will ordinarily range between 15 and 25 hours.
- Heating to a temperature just above the eutectoid temperature, and then either cooling very slowly in the furnace or holding at the same temperature.
- Heating and cooling alternately within about ±50°C (122°F) of the eutectoid temperature.
To some degree, the rate at which spheroidite forms depends on prior microstructure. For example, it is slowest for pearlite, and the finer the pearlite, the more rapid the rate. Also, prior cold work increases the spheroidizing reaction rate[3]
Spheroidization
[edit]No theory can precisely describe the process of spheroidization, and thus two different theories are adopted to explain the earlier and later stage of spheroidization respectively.
Rayleigh's Perturbation Theory
[edit]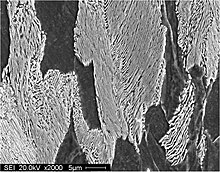
The original Rayleight theory deals with the instability of a rod-like shape caused by capillarity-induced perturbation. The same argument can be applied to pearlite spheroidization to explain certain phenomenons observed. Very few cementite particles appear with a rod-like shape in the early and middle stages of spheroidization. However, due to the anisotropic growth behavior of holes and fissures, it is frequently observed, after a long time, that large lamellar platelets do break up into long narrow ribbons and that the cementite ribbons gradually change their shapes and become long arrow rods via α-Fe3C interface diffusion. These long rods of cementite are relatively unstable with respect to capillarity-induced perturbation and tend to break up into a series of small spheroids[4].
Fault Migration Theory
[edit]
The contributions of Rayleight's perturbation theory are only limited to the later stage of spheroidization. Therefore there must be another mechanism which plays a more important role in the whole process of spheroidization. In most regions of perfect lamellar structure, the flat cementite plates are relatively stable. Certain regions with high interface curvature or imperfections , however, tend to trigger considerable spheroidization. The term "fault" in this case refers to terminations, kinks, holes, and fissures in these specific regions[4].
Spheroidization initiates and develops from lamellar faults. The driving force is the chemical potential gradient between the highly curved faults and the neighboring flat surface. The morphological changes which occur during spheroidization can be understood in terms of the recession of lamellar terminations, the expansion of holes, the growth of fissures, and the thickening of lamellae. On a two-dimensional polished section, these changes appear as a reduction in lamellar length and an increase in lamellar thickness. The continuous growth of holes and fissures leads to the connection of their profiles and to the break-up of large cementite platelets into small particles. Due to Ostwald ripening, large cementite particles become larger at the expense of smaller ones. All the cementite particles gradually evolve into spheroids[4].
Maity’s Mixed Mechanism of Spheroidization
[edit]Recently, Joydeep Maity and co-researchers applied a typical cyclic heat treatment around A3 on homogenizing annealed 0.6 wt.% carbon steel that has greatly accelerated the spheroidization process [5]. The cyclic heat treatment involved repeated short duration (6 min) holding above A3 followed by forced air cooling. The spheroidization process achieved completion only in 1 h 20 min with 8 cycles of heat treatment. A mixed mechanism of spheroidization has been proposed by Maity et al. [6] that involves simultaneous occurrence of thermal grooving, modified capillarity induced perturbation and lamellar thickening processes (requiring shorter diffusion distance) being aided by higher atomic mobility above A3 and generation of potential sites of lamellar faults in each cycle so as to cause rapid breakdown of lamellar pearlite into spheroids of cementite.
Accelerated Spheroidization
[edit]The conventional spheroidization process of steel consists of subcritical annealing treatment, which consumes significantly more energy and time than other steel phases. It has been a challenge for the steel industry to accelerate the spheroidization of pearlitic structure[5].
One method to accelerate spheroidization involves austenitizing, followed by rapid quenching to a temperature slightly above the martensitic transformation temperature, and up-quenching to a temperature slightly below 700°C (1300°F) for isothermal annealing. The supercooled austenite possesses large dislocation density and during subcritical annealing just below 700°C (1300°F), these dislocations aid the nucleation of cementite particles. This annealing process causes a rapid spheroidization through the abnormal decomposition of austenite. Research has shown that the austenite containing fine particles of carbides may undergo divorced eutectoid tranformation reaction, which produces a spheroidized structure in steel for austenitization at temperatures of 830°C (1530°F) and below[5]. In a recent work by Joydeep Maity and co-researchers, an investigation was carried out on the mechanism of accelerated spheroidization under cyclic heat treatment involving repeated short duration holding above A3 followed by forced air cooling of 0.6 wt.% carbon steel. Thermal grooving, modified capillarity induced perturbation and lamellar thickening were found to be the main processes responsible for rapid spheroidization. This was on contrary to the conventional subcritical spheroidization process where the termination migration process dominates the mechanism. This was attributed to the higher atomic mobility above A3 and the generation of potential diffusion sites of lamellar faults causing rapid breakdown of lamellar pearlite into spheroids of cementite even with short duration of holding in each cycle[6].
See Also
[edit]References
[edit]- ^ Callister Jr., W. D. 2007. Materials Science and Engineering—An Introduction, 7th ed. New York: Wiley. Glossary
- ^ Callister Jr., W. D. 2007. Materials Science and Engineering—An Introduction, 7th ed. New York: Wiley. p.329-330
- ^ a b Callister Jr., W. D. 2007. Materials Science and Engineering—An Introduction, 7th ed. New York: Wiley. p.390
- ^ a b c Y.L. Tian and R.W. Kraft, Mechanism of Pearlite Spheroidization,Metall. Trans. A, 1987, 18A, p 1412-1413
- ^ a b c Atanu Saha, Dipak Kumar Mondal, and Joydeep Maity. "An Alternate Approach to Accelerated Spheroidization in Steel by Cyclic Annealing." Journal of Materials Engineering and Performance, 2011, 20(1), p 114-119.
- ^ a b Joydeep Maity, Atanu Saha, Dipak Kumar Mondal and Koushik Biswas,“Mechanism of accelerated spheroidization of steel during cyclic heat treatment around upper critical temperature”,Philosophical Magazine Letters, Taylor & Francis; Vol. 93, Issue 4, 2013, pp 231-237. DOI:10.1080/09500839.2012.758390.
