User:Royhe62/sandbox
Inorganic synthesis
[edit]Inorganic synthesis and organometallic synthesis are used to prepare compounds with significant non-organic content. An illustrative example is the preparation of the anti-cancer drug cisplatin from potassium tetrachloroplatinate.[1]
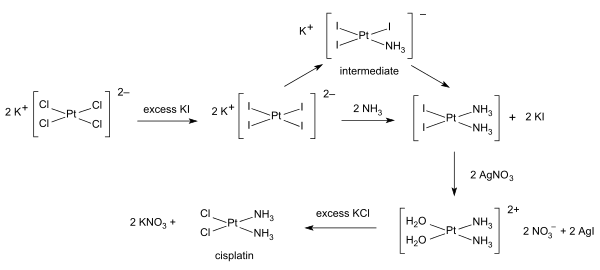
Inorganic Steps
[edit]Inorganic synthesis can be incredibly complex, and involve several intermediates and elementary steps. The steps outlined below are a few of the types of steps that can occur during an inorganic synthesis.
Oxidative Additiion
[edit]Oxidative Addition involves a metal center and a molecule with at least two atoms; the atoms can be of the same species, but do not necessarily have to be. The bond between the two atoms will break, and 2 new bonds will form between those atoms and the metal center. [2]

This is reffered to as "oxidative" because atoms A and B oxidize the metal; that is, the oxidation state of the metal is +2 relative to the oxidation state of the metal before the oxidative addition took place.[2]
Reductive Elimination
[edit]Reductive Elimination is the reverse reaction of Oxidative Addition. It invovles two atoms, A and B, bonded to a metal center. Reductive elimination will see atoms A and B form a bond with each other while both losing their bonds with the metal center.[3]
This is reffered to as "Reductive" because this reaction reduces the metla center; that is, the metal center's oxidation state will be 2 lower than it was before the reaction took place. [4]
Ligand Substitution
[edit]Ligand Substitution occurs when a chemical species attached the a metal center is replaced with a different one.
Associative Substitution
[edit]
Associative Substitution sees an incoming ligand Y coordinate to the metal center as the first step. Only after the association is complete, will the leaving ligand X leave the metal center, completing the substution process.[5]
This mechanism tends to occur with complexes that are unsaturated in both ligands and electrons; that is, ligands with <6 ligands and <18 valence electrons.[6]
The first step is generally rate determining, meaning that the reaction rate is second order; the reaction speed dependsd on both the concentration of the metal center and the concentration of the [Y] species.[7]
Dissociative Substitution
[edit]Dissociative substution sees the outgoing ligand X leave the metal center before the incoming lingad Y coordinates with the metal center. Only after X completely leaves does the new ligand Y coordinate to the metal center.
This mechanism tends to occur with ligands that are fully staurated; that is, complexes with 6 ligands and 18 valence electrons.
The rate determing step tends to be the dissociation step. Thus, the rate tends to be first order; the reaction rate depends on the concentration of the metal center, and is independant of both the concentration and identity of ligand Y.
- ^ Alderden, Rebecca A.; Hall, Matthew D.; Hambley, Trevor W. (1 May 2006). "The Discovery and Development of Cisplatin". J. Chem. Educ. 83 (5): 728. Bibcode:2006JChEd..83..728A. doi:10.1021/ed083p728.
- ^ a b Housecroft, Catherine E.; Sharpe, Alan G. (2018). Inorganic chemistry (Fifth edition ed.). Harlow, England London New York Boston San Francisco Toronto Sydney: Pearson. ISBN 978-1-292-13414-7.
{{cite book}}:|edition=has extra text (help) - ^ "Reductive elimination", Wikipedia, 2023-02-12, retrieved 2024-11-05
- ^ "Reductive elimination", Wikipedia, 2023-02-12, retrieved 2024-11-05
- ^ "Associative substitution", Wikipedia, 2022-03-08, retrieved 2024-11-05
- ^ "Associative substitution", Wikipedia, 2022-03-08, retrieved 2024-11-05
- ^ "Associative substitution", Wikipedia, 2022-03-08, retrieved 2024-11-05
