User:Publicscale/Octane
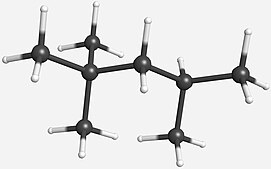
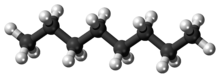
Octane is a hydrocarbon and an alkane with the chemical formula C8H18, and the condensed structural formula CH3(CH2)6CH3. Octane has many structural isomers that differ by the location of branching in the carbon chain. One of these isomers, 2,2,4-trimethylpentane (commonly called iso-octane), is used as one of the standard values in the octane rating scale.
Octane is a component of gasoline and petroleum. Under standard temperature and pressure, octane is an odorless, colorless liquid. Like other short-chained alkanes with a low molecular weight, it is volatile, flammable, and toxic. For examples, as a neurotoxin with narcotic effects, n-octane is almost twice times as toxic as n-heptane.[1]
Isomers
[edit]N-octane has 23 constitutional isomers. 8 of these isomers have one stereocenter; 3 of them have two stereocenters.

Achiral Isomers:
- 2-Methylheptane
- 4-Methylheptane
- 3-Ethylhexane
- 2,2-Dimethylhexane
- 2,5-Dimethylhexane
- (meso)-3,4-Dimethylhexane
- 3,3-Dimethylhexane
- 3-Ethyl-2-methylpentane
- 3-Ethyl-3-methylpentane
- 2,2,4-Trimethylpentane (i.e. iso-octane)
- 2,3,3-Trimethylpentane
- 2,3,4-Trimethylpentane
- 2,2,3,3-Tetramethylbutane
Chiral Isomers:
- (3R)-3-Methylheptane
- (3S)-3-Methylheptane
- (3R)-2,3-Dimethylhexane
- (3S)-2,3-Dimethylhexane
- (4R)-2,4-Dimethylhexane
- (4S)-2,4-Dimethylhexane
- (3R,4R)-3,4-Dimethylhexane
- (3S,4S)-3,4-Dimethylhexane
- (3R)-2,2,3-Trimethylpentane
- (3S)-2,2,3-Trimethylpentane
Octane in Culture
[edit]
"Octane" is colloquially used in the expression "high-octane".[2] The term is used to describe a powerful action because of the association with the concept of "octane rating". This is a misleading term, because the octane rating of gasoline is not directly related to the power output of an engine. Using gasoline of a higher octane than an engine is designed for cannot increase its power output.
Octane became well known in American popular culture in the 1960s, when gasoline companies boasted of "high octane" levels in their gasoline advertisements. The compound adjective "high-octane", meaning powerful or dynamic, is recorded in a figurative sense from 1944. By the 1990s, the phrase was commonly being used as a word intensifier, and it has found a place in modern English slang.
Difference between Octane and Octane Rating
[edit]Common Misconceptions on Octane Rating
[edit]
Due to its name, the chemical octane is often misunderstood as the only substance that determines the octane rating (or octane number) of a fuel. This is an inaccurate description. In reality, the octane rating is defined as a number describing the stability and ability of a fuel to prevent an engine from unwanted combustions[3] that occur spontaneously in the other regions within a cylinder (i.e. delocalized explosions from the spark plug). This phenomenon of combustion is more commonly known as engine knocking or self-ignition, which causes damage to pistons over time and reduces the lifespan of engines.
In 1927, Graham Edgar[4] devised the method of using iso-octane and n-heptane as reference chemicals, in order to rate the knock resistance of a fuel with respect to this isomer of octane,[5] thus the name "octane rating". By definition, the isomers iso-octane and n-heptane have an octane rating of 100 and 0, respectively.[6] Because of its more volatile nature, n-heptane ignites and knocks readily, which gives it a relatively low octane rating[7]; the isomer iso-octane causes less knocking because it is more branched and combusts more smoothly. In general, branched compounds with a higher intermolecular force (e.g. London dispersion force for iso-octane) will have a higher octane rating, because they are harder to ignite.[8]
Examples of misconceptions
[edit]There are numerous articles circulating the internet that propagate this misunderstanding. Below are some factually inaccurate statements.
Statement 1
[edit]When faced with the removal of lead as the primary octane provider in gasoline, refiners had two available alternatives, BTEX and ethanol....[5]
This statement is inaccurate, even though the alternatives indeed have a high octane rating. Alternative fuels do not contain octane, and cannot "provide" octane.
Statement 2
[edit]So, octane does not enhance the explosion in the cylinder like most people think... It just prevents the air-fuel mixture from igniting before the spark plug does it.[9]
While the first part of this statement is factual, the complete statement implies that octane is the only chemical contributing to octane rating, which is false.
Statement 3
[edit]You may be surprised by how much octane is in E85. Ethanol naturally has a high octane rating...[10]
This is a misleading statement. Octane rating is not necessarily (in fact, rarely) due to the presence of octane. The chemical ethanol itself has a high octane rating, resulting in high knock resistance in E85 fuels.
Octane Ratings of Octane Isomers
[edit]Octane isomers such as n-octane and 2,3,3-trimethylpentane have an octane rating of -20 and 106.1, respectively (RON measurement).[11] The large differences between the octane ratings for the isomers show that the compound octane itself is clearly not the only factor that determines octane ratings, especially for commercial fuels consist of a wide variety of compounds.
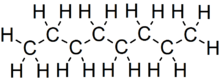
| |
| |
| |
| Names | |
|---|---|
| Systematic IUPAC name
Octane[12] | |
| Other names
n-Octane
| |
| Identifiers | |
3D model (JSmol)
|
|
| 3DMet | |
| 1696875 | |
| ChEBI | |
| ChEMBL | |
| ChemSpider | |
| DrugBank | |
| EC Number |
|
| 82412 | |
| KEGG | |
| MeSH | octane |
PubChem CID
|
|
| RTECS number |
|
| UNII | |
| UN number | 1262 |
| |
| |
| Properties | |
| CH3(CH2)6CH3 | |
| Molar mass | 114.232 g·mol−1 |
| Appearance | Colourless liquid |
| Odor | Gasoline-like[13] |
| Density | 0.703 g/cm3 |
| Melting point | −57.1 to −56.6 °C; −70.9 to −69.8 °F; 216.0 to 216.6 K |
| Boiling point | 125.1 to 126.1 °C; 257.1 to 258.9 °F; 398.2 to 399.2 K |
| 0.007 mg/dm3 (at 20 °C) | |
| log P | 4.783 |
| Vapor pressure | 1.47 kPa (at 20.0 °C) |
Henry's law
constant (kH) |
29 nmol/(Pa·kg) |
| Conjugate acid | Octonium |
| −96.63·10−6 cm3/mol | |
Refractive index (nD)
|
1.398 |
| Viscosity |
|
| Thermochemistry | |
Heat capacity (C)
|
255.68 J/(K·mol) |
Std molar
entropy (S⦵298) |
361.20 J/(K·mol) |
Std enthalpy of
formation (ΔfH⦵298) |
−252.1 to −248.5 kJ/mol |
Std enthalpy of
combustion (ΔcH⦵298) |
−5.53 to −5.33 MJ/mol |
| Hazards | |
| GHS labelling: | |
   
| |
| Danger | |
| H225, H304, H315, H336, H410 | |
| P210, P261, P273, P301+P310, P331 | |
| NFPA 704 (fire diamond) | |
| Flash point | 13.0 °C (55.4 °F; 286.1 K) |
| 220.0 °C (428.0 °F; 493.1 K) | |
| Explosive limits | 0.96 – 6.5% |
| Lethal dose or concentration (LD, LC): | |
LDLo (lowest published)
|
428 mg/kg (mouse, intravenous)[15] |
| NIOSH (US health exposure limits): | |
PEL (Permissible)
|
TWA 500 ppm (2350 mg/m3)[13] |
REL (Recommended)
|
TWA 75 ppm (350 mg/m3) C 385 ppm (1800 mg/m3) [15-minute][13] |
IDLH (Immediate danger)
|
1000 ppm[13] |
| Related compounds | |
Related alkanes
|
|
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
| |
Production and Use
[edit]Industrial Production
[edit]Like other alkanes, octane is commonly synthesised by industrial plants in large scales, which can be broken down into 3 steps.
From a petroleum or natural gas source, different alkanes are extracted at specific temperature and pressure, due to their different physical properties.
Shorter hydrocarbons (e.g. ethane, butane, iso-butene) can be combined to form longer hydrocarbons like octane. A common example is the alkylation reaction between iso-butane and 1-butene, which forms iso-octane.[17]
Through isomerisation, different isomers of octane can be produced.
Laboratory Use
[edit]Octane is not only found in gasoline, but it is also commonly used as a solvent in paints and adhesives. Moreover, it can be used an organic, non-polar solvent for various reactions, such as selective alkylations and hydrogenations.[18]
Because of octane's role in metabolism[19], the mechanism of its breakdown and oxidation is studied in biochemical labs, especially in organisms such as rats[20] and E. Coli[21].
References
[edit]- ^ "1988 OSHA PEL Project - Octane | NIOSH | CDC". www.cdc.gov. 2020-02-27. Retrieved 2024-04-19.
- ^ "Definition of HIGH-OCTANE". www.merriam-webster.com. 2024-04-13. Retrieved 2024-04-15.
- ^ "Dictionary.com | Meanings & Definitions of English Words". Dictionary.com. Retrieved 2024-04-03.
- ^ Beatty, Harold A. (1956-03-02). "Graham Edgar, Chemist of Parts". Science. 123 (3192): 365–365. doi:10.1126/science.123.3192.365. ISSN 0036-8075.
- ^ a b "Fact Sheet | A Brief History of Octane in Gasoline: From Lead to Ethanol | White Papers | EESI". www.eesi.org. Retrieved 2024-04-03.
- ^ "Octane". www.mckinseyenergyinsights.com. Retrieved 2024-04-03.
- ^ "Chemical isomer - Energy Education". energyeducation.ca. Retrieved 2024-04-03.
- ^ "Why do highly branched alkanes have higher octane numbers than their corresponding linear isomer?". Chemistry Stack Exchange. Retrieved 2024-04-15.
- ^ "What Does Octane Do In Gasoline? Octane Ratings". www.bellperformance.com. Retrieved 2024-04-04.
- ^ "What Is In Gasoline Vs E85 Flex Fuel". USA English. Retrieved 2024-04-05.
- ^ Balaban, A. T. (1983-01-01). "Topological indices based on topological distances in molecular graphs". Pure and Applied Chemistry. 55 (2): 199–206. doi:10.1351/pac198855020199. ISSN 1365-3075.
- ^ "octane - Compound Summary". PubChem Compound. USA: National Center for Biotechnology Information. 16 September 2004. Identification and Related Records. Retrieved 6 January 2012.
- ^ a b c d NIOSH Pocket Guide to Chemical Hazards. "#0470". National Institute for Occupational Safety and Health (NIOSH).
- ^ Dymond, J. H.; Oye, H. A. (1994). "Viscosity of Selected Liquid n-Alkanes". Journal of Physical and Chemical Reference Data. 23 (1): 41–53. Bibcode:1994JPCRD..23...41D. doi:10.1063/1.555943. ISSN 0047-2689.
- ^ "Octane". Immediately Dangerous to Life or Health Concentrations (IDLH). National Institute for Occupational Safety and Health (NIOSH).
- ^ "Fractionation". www.appliedcontrol.com. Retrieved 2024-04-19.
- ^ Ross, Julian (1986-01). "Ullman's encyclopedia of industrial chemistry". Applied Catalysis. 27 (2): 403–404. doi:10.1016/s0166-9834(00)82943-7. ISSN 0166-9834.
{{cite journal}}: Check date values in:|date=(help) - ^ "MilliporeSigma". Retrieved 2024-04-19.
- ^ "Metabolism of Alkanes and Fatty Acids". eQuillibrator. Retrieved 2024-04-19.
- ^ Olson, C. T.; Yu, K. O.; Hobson, D. W.; Serve, M. P. (1986-05). "The metabolism of n-octane in Fischer 344 rats". Toxicology Letters. 31 (2): 147–150. doi:10.1016/0378-4274(86)90008-1. ISSN 0378-4274. PMID 3715925.
{{cite journal}}: Check date values in:|date=(help) - ^ Gudiminchi, Rama Krishna; Randall, Charlene; Opperman, Diederik J.; Olaofe, Oluwafemi A.; Harrison, Susan T. L.; Albertyn, Jacobus; Smit, Martha S. (2012-12). "Whole-cell hydroxylation of n-octane by Escherichia coli strains expressing the CYP153A6 operon". Applied Microbiology and Biotechnology. 96 (6): 1507–1516. doi:10.1007/s00253-012-3984-5. ISSN 1432-0614. PMID 22410745.
{{cite journal}}: Check date values in:|date=(help)