User:Nrauto/Invasive carcinoma of no special type
| This is the sandbox page where you will draft your initial Wikipedia contribution.
If you're starting a new article, you can develop it here until it's ready to go live. If you're working on improvements to an existing article, copy only one section at a time of the article to this sandbox to work on, and be sure to use an edit summary linking to the article you copied from. Do not copy over the entire article. You can find additional instructions here. Remember to save your work regularly using the "Publish page" button. (It just means 'save'; it will still be in the sandbox.) You can add bold formatting to your additions to differentiate them from existing content. |
Article Draft
[edit]Lead
[edit]Invasive carcinoma of no special type (NST) is also referred to as invasive ductal carcinoma or infiltrating ductal carcinoma (IDC) and invasive ductal carcinoma, not otherwise specified (NOS). Each of these terms represents to the same disease entity, but for international audiences this article will use invasive carcinoma NST because it is the preferred term of the World Health Organization (WHO).[1][2][3]
Invasive carcinoma NST accounts for half of all breast cancer diagnoses in women[4] and is the most common type of invasive breast cancer.[5] It is also the most commonly diagnosed form of male breast cancer.[6] Invasive carcinoma NST is classified by its microscopic, molecular, and genetic features. Microscopically it is a breast carcinoma originating from the breast ducts that shows invasive features but lacks the "specific differentiating features" of other types of invasive breast cancers.[7][8][5] Invasive carcinoma NST is a diagnosis of exclusion, which means that for the diagnosis to be made all the other specific types must be ruled out. There are several rare sub-types of invasive carcinoma NST including pleomorphic carcinoma, carcinoma with osteoclast-like stromal giant cells, carcinoma with choriocarcinomatous features, and carcinoma with melanotic features.[1]
Epidemiology
[edit]Invasive carcinoma NST is one of the most common types of all breast cancers, accounting for 55% of breast cancer incidence.[4] Of the invasive breast cancers, invasive carcinoma NST accounts for up to 75% of cases.[5][8]
The incidence of ductal carcinomas as a whole is 86.3 cases per 100,000 women, with the incidence increasing sharply for women over 40 years of age and peaking at 285.6 cases per 100,000 for women between 70-79. This incidence has decreased slightly over time.[4]
Terminology
[edit]Invasive carcinoma NST is a type of breast cancer. It is one of the invasive breast cancers that originates from the breast ductal system, so that it is a type of ductal carcinoma. A defining feature of this ductal carcinoma is that it lacks the "specific differentiating features" of other types of ductal carcinomas. It is important to note that IDC, invasive ductal carcinoma NOS, and invasive carcinoma NST all refer to the same type of breast cancer. For consistency and to serve an international audience, this article will use invasive ductal NST.
The terminology of invasive carcinoma NST has undergone recent change. Differing opinions within the medical and public health communities have lead to some variance in how this disease is referred in research and clinical settings.
In 2012 the International Agency for Research on Cancer (IARC), a sub-department of the WHO, published the 4th edition of the WHO Classification of Tumors of the Breast. Previously known as 'invasive ductal carcinoma, not otherwise specified', these most recent guidelines advocated for the use of 'invasive carcinoma of no special type'.[2][1]
There are, however, differing opinions and practices. The research literature continues to use IDC or invasive ductal carcinoma NOS,[9][10] and some medical textbooks have offered support for continued use of IDC or IDC/NOS.[11]
Signs and symptoms
[edit]In most cases breast cancers are asymptomatic and are detected by routine clinical screening exams (?, citation). In about 30% of cases a breast mass may be felt.[12][9] The mass will not fluctuate with the menstrual period.[13] Changes to the overlying skin including dimpling, pinching, orange peel-like texture, or nipple retraction may be seen.[14] Non-healing ulcers can form in advanced disease, and were more common historically prior to modern medical care.
Metastatic lesions from breast cancer may produce symptoms according to that organ system. The most common sites for metastasis are the bone, lung, liver, and brain.[15] Skin metastases most commonly extend to the skin overlying the mass, but may spread to the axilla or more distant areas.[16] Metastasis to adjacent lympatics may produce palpable masses in the axilla or an orange peel-like texture of the skin of the effected breast.[16][17]

Diagnosis
[edit]

The process of diagnosing invasive carcinoma NST is similar to that of other breast cancers. The process may be prompted by a patient presenting with a palpable mass or by evidence of a suspicious lesion on routine screening tests.[18] Tissue sampling is required for complete classification which will help determine prognosis and treatment plan. Tissue samples will be looked at under the microscope for histopathological type, grade, and stage (TNM). Immunohistochemical staining is used to establish receptor status, and the presence or absence of pertinent genes is determined by DNA testing.
This article will discuss the features specific to invasive carcinoma NST. More general and complete discussions can be found in articles on breast cancer screening and breast cancer classification.
Histopathologic criteria
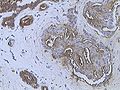
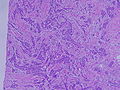
[edit]On microscopic evaluation carcinomatous cells are seen below the basement membrane of lactiferous ducts and invade into the surrounding breast stroma. Otherwise, there are no specific histologic characteristics, essentially making it a diagnosis of exclusion.[19] The histopathologic characteristics seen in these lesions are heterogenous, and may include "diffuse sheets, nests, cords, or singly distributed cells with variable amount of ductal differentiation. The amount of ductal differentiation ranges from more than 70% of tumor tissue to complete absence. Tumor cells are pleomorphic, vary in shape and size, and are usually with prominent nucleoli and numerous mitoses. The areas of necrosis and calcification can be detected in 60% of cases. Foci of squamous metaplasia, apocrine metaplasia, or clear cell changes are sometimes present. The amount of stroma is variable, ranging from none to abundant."[20][21]
Small inclusions of special features may be present within a icnst tissue sample, but will be 'limited' (ie <10%). Carcinomas of mixed type will have a specialized pattern or lobular carcinoma in the majority (ie at least 50%) of the tumor and a non-specialized pattern in between 10-49% of the sample. So will be called mixed invasive NST and special type or mixed invasive NST and lobular carcinoma.[7]
-
Invasive ductal carcinoma of the breast assayed with anti Mucin 1 antibody.
-
Breast cancer (Infiltrating ductal carcinoma of the breast) assayed with anti HER-2 (ErbB2) antibody.
-
Histopathology of invasive ductal carcinoma of the breast representing a scirrhous growth. Core needle biopsy. Hematoxylin and eosin stain.
-
Invasive ductal carcinoma of the breast. H&E stain.
-
Histopathology of invasive ductal carcinoma of the breast representing a scirrhous growth. Core needle biopsy. HER-2/neu oncoprotein expression by Ventana immunostaining system.
-
Histopathology of invasive ductal carcinoma of the breast. H&E stain.
-
Invasive ductal carcinoma, with occasional entrapped normal ducts (arrow)
-
Invasive ductal carcinoma of the breast assayed with anti Mucin 1 antibody.
-
Breast cancer (Infiltrating ductal carcinoma of the breast) assayed with anti HER-2 (ErbB2) antibody.
-
Histopathology of invasive ductal carcinoma of the breast representing a scirrhous growth. Core needle biopsy. Hematoxylin and eosin stain.
-
Invasive ductal carcinoma of the breast. H&E stain.
-
Histopathology of invasive ductal carcinoma of the breast representing a scirrhous growth. Core needle biopsy. HER-2/neu oncoprotein expression by Ventana immunostaining system.
-
Histopathology of invasive ductal carcinoma of the breast. H&E stain.
-
Invasive ductal carcinoma, with occasional entrapped normal ducts (arrow)
Staging
[edit]Cancers in general will be staged according their degree of tumor size, lymph node involvement, and evidence of metastasis. There are two types, clinical staging and pathologic staging. Clinical staging uses information derived from physical examination, clinical imaging, and biopsy. Pathologic staging takes place after the tumor is removed surgically, when a pathologist is able to make more direct measurements of the tumor characteristics. Pathologic staging is considered more accurate, but clinical staging can give useful information to determine treatment plans prior to surgical efforts. Both clinical and pathologic staging use the TNM staging system, which take into account the tumor size (T), lymph node involvement (N), and evidence of metastasis (M). The TNM staging system designed for breast cancer is shown in the table below.[22]
Tumor size
[edit]In clinical staging, tumor size is determined by clinical imaging. A more accurate measurement of tumor size and observation of extension into adjacent structures can be determined via pathological staging following surgery.
Lymph node involvement
[edit]Absence of cancer cells in the lymph nodes is a good indication that the cancer has not spread systemically. Presence of cancer in the lymph nodes indicates the cancer may have spread. In studies, some women have had presence of cancer in the lymph nodes, were not treated with chemotherapy, and still did not have a systemic spread. Therefore, lymph node involvement is not an absolute predictor of spread.[23]
| Description | |
|---|---|
| Primary tumor (T) | T1=tumor size ≤20 mm
T2=>20 mm but ≤50 mm T3=>50 mm T4=tumor of any size with direct extension to the chest wall and/or skin |
| Regional lymph nodes (N) | N0=no regional lymph node metastases
N1mi=micrometastases N1=metastases to moveable ipsilateral axillary lymph nodes N2=metastases in ipsilateral axillary lymph nodes that are clinically fixed N3=metastases that are more extensive |
| Distant metastasis (M) | M0=no evidence of distant metastases
M1=distant detectable metastases as determined by clinical and radiographic means |
| Stage | |
| 0 | DCIS |
| I | IA=T1, N0, M0
IB=T0, N1mi, M0 or T1, N1mi, M0 |
| II | IIA=T0, N1, M0 or T1, N1, M0 or T2, N0, M0
IIB=T2, N1, M0 or T3, N0, M0 |
| III | Larger size tumors with various combinations of lymph node involvement that are more extensive than stage II, but no distant metastases |
| IV | Distant metastases (M1) |
Grading
[edit]The appearance of cancer cells under a microscope is another predictor of systemic spread. The more different the cancer cells look compared to normal duct cells, the greater the risk of systemic spread. There are three characteristics that differentiate cancer cells from normal cells.
- Tendency to form tubular structures
- Nuclear size, shape, and staining intensity
- Mitotic rate - Rate of cell division
The histologic appearance of cancer cells can be scored on these three parameters on a scale from one to three. The sum of these grades is a number between 3 and 9. The score is called a Bloom Richardson Grade (BR) and is expressed [sum of the grades]/9. For example, cells that were graded 2 on all three parameters would result in a BR score of 6/9.
A score of 5 and under is considered low. 6 to 7 is considered intermediate. 8 to 9 is considered high.[23]
Vascular invasion
[edit]The presence of cancer cells in small blood vessels is called vascular invasion. The presence of vascular invasion increases the probability of systemic spread.[23]
DNA analysis
[edit]DNA analysis indicates the amount of DNA in cancer cells and how fast the cancer is growing.
Cells with the normal amount of DNA are called diploid. Cells with too much or too little DNA are called aneuploid. Aneuploid cells are more likely to spread than diploid cells.
DNA testing indicates the rate of growth by determining the number of cells in the synthetic phase (S Phase). An S Phase > 10% means a higher chance of spreading.
The results of DNA testing are considered less reliable predictors of spread than size, histology, and lymph node involvement.
On a mammogram, it is usually visualized as a mass with fine spikes radiating from the edges. On physical examination, this lump usually feels much harder or firmer than benign breast lesions such as fibroadenoma. On microscopic examination, the cancerous cells invade and replace the surrounding normal tissues. IDC is divided in several histological subtypes.
Annotations
[edit]### classification
*invasive ductal carcinoma; IDC, not otherwise specified; invasive carcinoma of no special type (WHO 2012)
* phenotypical/histological, molecular, genetic
* IDC is historically and currently the most commonly used term for this type of breast cancer. WHO 2012, however, would like to do away with IDC in favor of ICNST because “the use of the term ‘ductal' perpetuates the traditional but incorrect concept that these tumors are derived exclusively from mammary ductal epithelium in distinction from lobular carcinomas, which were deemed to have arisen from within lobules, for which there is also no evidence." (2012 World Health Organization (WHO) Classification of Breast Tumors) Professionals/experts may prefer IDC over ICNST (Rosen's Breast Pathology 5th, ch 12).
* Terminology of breast cancers may appear ambiguous to casuals, 1) as more research has delineated types, and 2) professional organizations have differing opinions on most accurate names.
* Ductal carcinomas are 75% of mammary carcinomas, lobular makes up a big chunk of the other 25%. Ductal carcinomas as a group include IDC (also known as IDC/NOS or ICNST, heretoafter referred to as IDC) and ductal carcinomas with special defining characteristics such tubular, medullary, metaplastic, adenoid cystic, papillary, and mucinous. This article discusses IDC
* You call it IDC if it has a glob of invasive cells with <10% (ie "limited") foci of characteristics of tubular, medullary, mucinous, or papillary; lobular carcinoma; DCIS
* "Tumors with such combined morphology can either exhibit “hybrid” morphology or show a “mixed” pattern of two (or more) well-defined histologic types.
* "prognosis is likely to be that of the dominant component"
* "In one detailed review of 1,000 carcinomas, approximately one-third of the lesions characterized as IDC expressed one or more combined features.... 140 tumors believed to have “mixed” morphology based on histologic examination alone constituted 3.6% of all cases studied.
* need to reassess carcinomas classified as IDC of the mixed type with the growing number of markers associated with specific types of carcinoma in an effort to develop a more meaningful subclassification
* The 4th edition of the WHO Classification of Tumors of the Breast (A Brief Overview of the WHO Classification of Breast Tumors, 4th Edition, Focusing on Issues and Updates from the 3rd Edition)
* let's change it from IDC NOS to ICNST
* the argument is that 1) "ductal" is an assumption about the origin of the tumor cells that doesn't have definitive proof, 2) IDC NOS isn't even a uniform group, and 3) "‘ductal’ does not represent a distinguishing pathological feature for breast cancers of no specific or of specific type"
* "The diagnosis is made by exclusion of recognized specific types of breast cancers."
* "Carcinomas of mixed type have a specialized pattern in at least 50% of the tumor and a non-specialized pattern in between 10% and 49%."
* "Rare morphological variants of invasive carcinoma NST include pleomorphic carcinoma, carcinoma with osteoclast-like stromal giant cells, carcinoma with choriocarcinomatous features, and carcinoma with melanotic features."
* "The most common specific subtypes include invasive lobular, tubular, cribriform, metaplastic, apocrine, mucinous, papillary, and micropapillary carcinoma, as well as carcinoma with medullary, neuroendocrine, and salivary gland/skin adnexal type features."
* "Invasive ductal carcinoma (IDC) is the most common form of invasive breast cancer. It accounts for 55% of breast cancer incidence upon diagnosis." (Eheman CR, Shaw KM, Ryerson AB, Miller JW, Ajani UA, White MC. The changing incidence of in situ and invasive ductal and lobular breast carcinomas. Cancer Epidemiol Biomarkers Prev. 2009;18(6):1763–9.)
* "Cancers originating from the ducts are known as ductal carcinomas, while those originating from the lobules are known as lobular carcinomas. However, it is now found that this sort of tumor growth variation is not related to the site or the cell of origin, but there could be differences in tumor cell biology: whether the tumor cells express E-cadherin or not." (Robbins 8th ed)
invaded through the basement membrane of ductule into breast stroma.
* For the morphological study of breast carcinoma, two main questions should be answered: is the tumor limited to the epithelial component of the breast (in situ carcinoma) or has invaded the stroma to become invasive carcinoma and does the tumor arise from the duct (ductal carcinoma) or from the lobule (lobular carcinoma)? (Diversity of Breast Carcinoma: Histological Subtypes and Clinical Relevance; jaffar makki)
* "Some of them have enough distinctive features and particular behavior to be classified as special subtypes, while the majority, which constitute about 75% of IDC, fail to exhibit sufficient morphological features to be classified as specific histological types and are generally designated as IDC not otherwise specified (NOS)."
* "IDC no specific type (NST) is the most common type of IDC that constitutes about 40%–75% of all mammary invasive carcinomas in the published series." (15. Farid M. Essentials of Diagnostic Breast Pathology, Practical Approach. 1st ed. Berlin: Springer; 2007.)
*
* 1. Lakhani SR, Ellis IO, Schnitt SJ, Tan PH, Van de Vijver MJ, editors. WHO Classification of Tumours of the Breast. Fourth ed. IARC; Lyon: 2012. ISBN.13.
*
References
[edit]- ^ a b c WHO classification of tumours of the breast. Sunil R. Lakhani, International Agency for Research on Cancer, World Health Organization. Lyon. 2012. ISBN 978-92-832-4488-2. OCLC 956377388.
{{cite book}}: CS1 maint: location missing publisher (link) CS1 maint: others (link) - ^ a b Sinn, Hans-Peter; Kreipe, Hans (2013). "A Brief Overview of the WHO Classification of Breast Tumors, 4th Edition, Focusing on Issues and Updates from the 3rd Edition". Breast Care. 8 (2): 149–154. doi:10.1159/000350774. ISSN 1661-3791.
- ^ Hoda, Syed A.; Kaplan, Rachel E. (2013-02). "World Health Organization (WHO) Classification of Breast Tumours, 4th ed". American Journal of Surgical Pathology. 37 (2): 309–310. doi:10.1097/pas.0b013e318273b19b. ISSN 0147-5185.
{{cite journal}}: Check date values in:|date=(help) - ^ a b c Eheman, Christie R.; Shaw, Kate M.; Ryerson, Aliza Blythe; Miller, Jacqueline W.; Ajani, Umed A.; White, Mary C. (2009-06-01). "The Changing Incidence of In situ and Invasive Ductal and Lobular Breast Carcinomas: United States, 1999-2004". Cancer Epidemiology, Biomarkers & Prevention. 18 (6): 1763–1769. doi:10.1158/1055-9965.epi-08-1082. ISSN 1055-9965.
- ^ a b c Farid., Moinfar, (2007). Essentials of diagnostic breast pathology : a practical approach ; with 6 tables. Springer. ISBN 978-3-540-45117-4. OCLC 634182636.
{{cite book}}: CS1 maint: extra punctuation (link) CS1 maint: multiple names: authors list (link) - ^ Zheng, Guoliang; Leone, Jose Pablo (2022-05-24). "Male Breast Cancer: An Updated Review of Epidemiology, Clinicopathology, and Treatment". Journal of Oncology. 2022: e1734049. doi:10.1155/2022/1734049. ISSN 1687-8450. PMC 9155932. PMID 35656339.
{{cite journal}}: CS1 maint: PMC format (link) CS1 maint: unflagged free DOI (link) - ^ a b Sinn HP, Kreipe H (May 2013). "A Brief Overview of the WHO Classification of Breast Tumors, 4th Edition, Focusing on Issues and Updates from the 3rd Edition". Breast Care. 8 (2): 149–154. doi:10.1159/000350774. PMC 3683948. PMID 24415964.
- ^ a b Makki, Jaafar (2015-01). "Diversity of Breast Carcinoma: Histological Subtypes and Clinical Relevance". Clinical Medicine Insights: Pathology. 8: CPath.S31563. doi:10.4137/cpath.s31563. ISSN 1179-5557.
{{cite journal}}: Check date values in:|date=(help) - ^ a b Watkins, Elyse J. (2019-10). "Overview of breast cancer". JAAPA. 32 (10): 13–17. doi:10.1097/01.JAA.0000580524.95733.3d. ISSN 1547-1896.
{{cite journal}}: Check date values in:|date=(help) - ^ O'Connor, Dómhnall J.; Davey, Matthew G.; Barkley, Laura R.; Kerin, Michael J. (2022-02). "Differences in sensitivity to neoadjuvant chemotherapy among invasive lobular and ductal carcinoma of the breast and implications on surgery–A systematic review and meta-analysis". The Breast. 61: 1–10. doi:10.1016/j.breast.2021.11.017.
{{cite journal}}: Check date values in:|date=(help) - ^ Hoda, Syed A. (2021). Rosen's breast pathology Syed A. Hoda. Paul Peter Rosen (Fifth edition ed.). Philadelphia. ISBN 978-1-4963-9892-5. OCLC 1224297918.
{{cite book}}:|edition=has extra text (help)CS1 maint: location missing publisher (link) - ^ Torre, Lindsey A.; Bray, Freddie; Siegel, Rebecca L.; Ferlay, Jacques; Lortet-Tieulent, Joannie; Jemal, Ahmedin (2015-02-04). "Global cancer statistics, 2012". CA: A Cancer Journal for Clinicians. 65 (2): 87–108. doi:10.3322/caac.21262. ISSN 0007-9235.
- ^ Colledge NR, Walker BR, Ralston SH, Britton R, eds. (2010). Davidson's principles and practice of medicine (21st ed.). Edinburgh: Churchill Livingstone/Elsevier. ISBN 978-0-7020-3084-0.
- ^ Zhang, Bao-Ning; Cao, Xu-Chen; Chen, Jia-Yi; Chen, Jie; Fu, Li; Hu, Xi-Chun; Jiang, Ze-Fei; Li, Hong-Yuan; Liao, Ning; Liu, Dong-Geng; Tao, Ouyang; Shao, Zhi-Min; Sun, Qiang; Wang, Shui; Wang, Yong-Sheng (2012-05). "Guidelines on the diagnosis and treatment of breast cancer (2011 edition)". Gland Surgery. 1 (1): 391–361. doi:10.3978/j.issn.2227-684X.2012.04.07. ISSN 2227-8575. PMC 4115707. PMID 25083426.
{{cite journal}}: Check date values in:|date=(help)CS1 maint: PMC format (link) - ^ Chen, Meng-Ting; Sun, He-Fen; Zhao, Yang; Fu, Wen-Yan; Yang, Li-Peng; Gao, Shui-Ping; Li, Liang-Dong; Jiang, Hong-lin; Jin, Wei (2017-08-23). "Comparison of patterns and prognosis among distant metastatic breast cancer patients by age groups: a SEER population-based analysis". Scientific Reports. 7 (1): 9254. doi:10.1038/s41598-017-10166-8. ISSN 2045-2322. PMC 5569011. PMID 28835702.
{{cite journal}}: CS1 maint: PMC format (link) - ^ a b Milam, Emily C.; Rangel, Lauren K.; Pomeranz, Miriam K. (2020-11-23). "Dermatologic sequelae of breast cancer: From disease, surgery, and radiation". International Journal of Dermatology. 60 (4): 394–406. doi:10.1111/ijd.15303. ISSN 0011-9059.
- ^ Robbins basic pathology. Saunders/Elsevier. 2007. ISBN 978-0-8089-2366-4.
- ^ Mathis, Kellie L.; Hoskin, Tanya L.; Boughey, Judy C.; Crownhart, Brian S.; Brandt, Kathy R.; Vachon, Celine M.; Grant, Clive S.; Degnim, Amy C. (2010-03). "Palpable Presentation of Breast Cancer Persists in the Era of Screening Mammography". Journal of the American College of Surgeons. 210 (3): 314–318. doi:10.1016/j.jamcollsurg.2009.12.003. ISSN 1072-7515.
{{cite journal}}: Check date values in:|date=(help) - ^ Abdelmessieh P. "Breast Cancer Histology". Medscape. Retrieved 2019-10-04. Updated: May 24, 2018
- ^ Makki, Jaafar (2015-01). "Diversity of Breast Carcinoma: Histological Subtypes and Clinical Relevance". Clinical Medicine Insights: Pathology. 8: CPath.S31563. doi:10.4137/cpath.s31563. ISSN 1179-5557.
{{cite journal}}: Check date values in:|date=(help) - ^ Jaworski, Richard (2004-12). "Rosai and Ackerman's Surgical Pathology: Ninth Edition". Pathology. 36 (6): 595. doi:10.1080/00313020400010906. ISSN 0031-3025.
{{cite journal}}: Check date values in:|date=(help) - ^ author., Nelson, Heidi D.,. Screening for breast cancer : a systematic review to update the 2009 U.S. Preventive Services Task Force recommendation. OCLC 948775981.
{{cite book}}:|last=has generic name (help)CS1 maint: extra punctuation (link) CS1 maint: multiple names: authors list (link) - ^ a b c Link J. The Breast Cancer Survival Manual (4th ed.).