User:NatMath13/Transition metal carbene complex
| This is the sandbox page where you will draft your initial Wikipedia contribution.
If you're starting a new article, you can develop it here until it's ready to go live. If you're working on improvements to an existing article, copy only one section at a time of the article to this sandbox to work on, and be sure to use an edit summary linking to the article you copied from. Do not copy over the entire article. You can find additional instructions here. Remember to save your work regularly using the "Publish page" button. (It just means 'save'; it will still be in the sandbox.) You can add bold formatting to your additions to differentiate them from existing content. |
Article Draft
[edit]Lead
[edit]A transition metal carbene complex is an organometallic compound featuring a divalent carbon ligand, itself also called a carbene.[1] Carbene complexes have been synthesized from most transition metals and f-block metals,[2] using many different synthetic routes such as nucleophilic addition and alpha-hydrogen abstraction.[1] The term carbene ligand is a formalism since many are not directly derived from carbenes and most are much less reactive than lone carbenes[2]. Described often as =CR2, carbene ligands are intermediate between alkyls (−CR3) and carbynes (≡CR). Many different carbene-based reagents such as Tebbe's reagent are used in synthesis. They also feature in catalytic reactions, especially alkene metathesis, and are of value in both industrial heterogeneous and in homogeneous catalysis for laboratory- and industrial-scale preparation of fine chemicals.[1][3][4]
Classification
[edit]Metal carbene complexes are often classified into two types. The Fischer carbenes, named after Ernst Otto Fischer, feature strong π-acceptors at the metal and are electrophilic at the carbene carbon atom. Schrock carbenes, named after Richard R. Schrock, are characterized by more nucleophilic carbene carbon centers; these species typically feature higher oxidation state (valency) metals. N-Heterocyclic carbenes (NHCs) were popularized following Arduengo's isolation of a stable free carbene in 1991.[5] Reflecting the growth of the area, carbene complexes are now known with a broad range of different reactivities and diverse substituents. Often it is not possible to classify a carbene complex solely with regards to its electrophilicity or nucleophilicity.[1]
Fischer carbenes
[edit]
The common features of Fisher carbenes are:[6]
- low oxidation state metal center
- middle and late transition metals Fe(0), Mo(0), Cr(0)
- π-acceptor metal ligands
- π-donor substituents on the carbene atom such as alkoxy and alkylated amino groups.
Examples include (CO)5W=COMePh and (OC)5Cr=C(NR2)Ph.

Fisher carbene complexes are related to the singlet form of carbenes, where both electrons occupy the same sp2 orbital at the carbon. This lone pair donates to a metal-based empty d orbital, forming a σ bond. π-backbonding from a filled metal d orbital to the empty p orbital of the carbon atom is possible. However this interaction is generally weak since the alpha donor atoms also donate to this orbital. As such, fisher carbenes are characterized as having partial double bond character. The major resonance structures of Fisher carbenes put the negative charge on the metal centre, and the positive on the carbon atom, making it electrophilic.[6]

Fischer carbenes can be likened to ketones, with the carbene carbon atom being electrophilic, like the carbonyl carbon atom of a ketone. This can be seen from the resonance structures, where there is a significant contribution from the structure bearing a positive carbon centre.[6] Like ketones, Fischer carbene species can undergo aldol-like reactions. The hydrogen atoms attached to the carbon atom α to the carbene carbon atom are acidic, and can be deprotonated by a base such as n-butyllithium, to give a nucleophile, which can undergo further reaction.[7]
Schrock carbenes
[edit]
Schrock carbenes do not have π-accepting ligands on the metal centre, and are typically found with:[6]
- high oxidation state metal center
- early transition metals Ti(IV), Ta(V)
- σ-donor and sometimes π-donor metal ligands
- hydrogen and alkyl substituents on carbenoid carbon.
Examples include ((CH3)3CCH2)Ta=CHC(CH3)3[9] and Os(PPh3)2(NO)Cl(=CH2)[10].

Bonding in such complexes can be viewed as the coupling of a triplet state metal and triplet carbene, forming a true double bond. Both the metal and carbon atom donate 2 electrons, one to each bond. Since there is no donation to the carbene atom from adjacent groups, the extent of pi backbonding is much greater, giving a strong double bond. These bonds are weakly polarized towards carbon and therefore the carbene atom is a nucleophile. Furthermore, the major resonance structures of Schrock carbene put the negative charge on the carbon atom, making it nucleophilic.[6] Complexes with the methylidene ligand (=CH2) are the simplest Schrock-type carbenes.

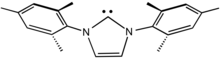
N-Heterocyclic carbenes
[edit]N-Heterocyclic carbenes (NHCs) are particularly common carbene ligands.[11] They are popular because they are more readily prepared than Schrock and Fischer carbenes. In fact, many NHCs are isolated as the free ligand, since they are persistent carbenes.[12][13] Being strongly stabilized by π-donating substituents, NHCs are powerful σ-donors but π-bonding with the metal is weak.[14] For this reason, the bond between the carbon and the metal center is often represented by a single dative bond, whereas Fischer and Schrock carbenes are usually depicted with double bonds to metal. Continuing with this analogy, NHCs are often compared with trialkylphosphine ligands. Like phosphines, NHCs serve as spectator ligands that influence catalysis through a combination of electronic and steric effects, but they do not directly bind substrates.[15][16]
Bimetallic carbene complexes
[edit]An early example of this bonding mode was provided by [C5Me5Mn(CO)2]2(μ−CO) prepared from diazomethane:
- 2 C5Me5Mn(CO)2(thf) + CH2N2 → [C5Me5Mn(CO)2]2(μ−CH2] + N2 + 2 thf
Another example of this family of compounds is Tebbe's reagent. It features a methylene bridge joining titanium and aluminum.[17]
Application of Metal Carbenes
[edit]Metal carbene complexes have applications in hetereogeneous and homogeneous catalysis, and as reagents for organic reactions.
Catalysis
[edit]
The main heterogeneous application of metal carbenes involves none of the above classes of compounds, but rather heterogeneous catalysts used for alkene metathesis for the synthesis of higher alkenes. A variety of related reactions are used to interconvert light alkenes, e.g. butenes, propylene, and ethylene.[18] Carbene complexes are invoked as intermediates in the Fischer–Tropsch route to hydrocarbons.[3]
A variety of homogeneous carbene catalysts, especially the Grubbs' ruthenium and Schrock molybdenum-imido catalysts have been used for olefin metathesis in laboratory-scale synthesis of natural products and materials science.[4]
Carbene Reagents
[edit]Carbonyl Olefination
[edit]Homogeneous Schrock-type carbene complexes such as Tebbe's reagent are also used for the olefination of carbonyls, replacing the oxygen atom with a methylidene group. The nucleophilic carbon atom behaves similarly to the carbon atom of the phosphorus ylide in the Wittig reaction, attacking the electrophilic carbonyl atom of a ketone, followed by elimination of a metal oxide.[1]

Methyl Abstraction
[edit]In the nucleophilic abstraction reaction, a methyl group can be abstracted from the donating group of a Fischer carbene, making it a strong nucleophile for further reaction.[6]

Carbene Insertion
[edit]Diazo compounds like methyl phenyldiazoacetate can be used for cyclopropanation or to insert into C-H bonds of organic substrates. These reactions are catalyzed by dirhodium tetraacetate or related chiral derivatives. Such catalysis is assumed to proceed via the intermediacy of carbene complexes.[19]

Wulff-Dötz Reaction
[edit]Fischer carbenes are used with alkynes as the starting reagents for the Wulff–Dötz reaction, forming phenols.[20]

History
[edit]
The first metal carbene complex to have been reported was Chugaev's red salt, first synthesized as early as 1925, although it was never identified to be a carbene complex.[21] The characterization of (CO)5W(COCH3(Ph)) in the 1960s is often cited as the starting point of the area and Ernst Otto Fischer, for this and other achievements in organometallic chemistry, was awarded the 1973 Nobel Prize in Chemistry.[22] In 1968, Hans-Werner Wanzlick and Karl Öfele separately reported metal-bonded N-heterocyclic carbenes[6][23][24]. The synthesis and characterization of ((CH3)3CCH2)Ta=CHC(CH3)3 by Richard R. Schrock in 1974 marked the first metal alkylidene complex.[9] In 1991, Anthony J. Arduengo synthesized and crystallized the first persistent carbene, an NHC with large adamantane alkyl groups, accelerating the field of N-heterocarbene ligands to its current use.[5][6]
References
[edit]- ^ a b c d e Elschenbroich, Christoph; Elschenbroich, Christoph (2011). Organometallics (3., compl. rev. and extended ed ed.). Weinheim: WILEY-VCH. ISBN 978-3-527-29390-2.
{{cite book}}:|edition=has extra text (help) - ^ a b Arnold, Polly L.; Casely, Ian J. (2009-08-12). "F-Block N-Heterocyclic Carbene Complexes". Chemical Reviews. 109 (8): 3599–3611. doi:10.1021/cr8005203. ISSN 0009-2665.
- ^ a b Herrmann, Wolfgang A. (1982-02). "Organometallic Aspects of the Fischer‐Tropsch Synthesis". Angewandte Chemie International Edition in English. 21 (2): 117–130. doi:10.1002/anie.198201171. ISSN 0570-0833.
{{cite journal}}: Check date values in:|date=(help) - ^ a b Fürstner, Alois (2000-09-01). "Olefin Metathesis and Beyond". Angewandte Chemie. 39 (17): 3012–3043. doi:10.1002/1521-3773(20000901)39:17<3012::AID-ANIE3012>3.0.CO;2-G.
- ^ a b Arduengo III AJ, Harlow RL, Kline M (1991). "A stable crystalline carbene". J. Am. Chem. Soc. 113 (1): 361–363. doi:10.1021/ja00001a054.
- ^ a b c d e f g h de Frémont, Pierre; Marion, Nicolas; Nolan, Steven P. (2009-04). "Carbenes: Synthesis, properties, and organometallic chemistry". Coordination Chemistry Reviews. 253 (7–8): 862–892. doi:10.1016/j.ccr.2008.05.018. ISSN 0010-8545.
{{cite journal}}: Check date values in:|date=(help) - ^ Crabtree RH (2005). The Organometallic Chemistry of the Transition Metals (4th ed.). New Jersey: Wiley-Interscience. ISBN 978-0-471-66256-3.
- ^ "A neutron diffraction study of bis(cyclopentadienyl)(methyl)(methylene)tantalum(V) at 15 K". Acta Crystallographica Section C Crystal Structure Communications. 44 (3): 439–443. 1988-03-15. doi:10.1107/S0108270187010527.
- ^ a b Schrock, Richard R. (1974-10). "Alkylcarbene complex of tantalum by intramolecular .alpha.-hydrogen abstraction". Journal of the American Chemical Society. 96 (21): 6796–6797. doi:10.1021/ja00828a061. ISSN 0002-7863.
{{cite journal}}: Check date values in:|date=(help) - ^ Hill, Anthony F.; Roper, Warren R.; Waters, Joyce M.; Wright, Anthony H. (1983-09). "A mononuclear, low-valent, electron-rich osmium methylene complex". Journal of the American Chemical Society. 105 (18): 5939–5940. doi:10.1021/ja00356a050. ISSN 0002-7863.
{{cite journal}}: Check date values in:|date=(help) - ^ Hahn FE, Jahnke MC (2008). "Heterocyclic carbenes: synthesis and coordination chemistry". Angewandte Chemie. 47 (17): 3122–72. doi:10.1002/anie.200703883. PMID 18398856.
- ^ Aldeco-Perez E, Rosenthal AJ, Donnadieu B, Parameswaran P, Frenking G, Bertrand G (October 2009). "Isolation of a C5-deprotonated imidazolium, a crystalline "abnormal" N-heterocyclic carbene". Science. 326 (5952): 556–9. Bibcode:2009Sci...326..556A. doi:10.1126/science.1178206. PMC 2871154. PMID 19900893.
- ^ Arduengo AJ, Goerlich JR, Marshall WJ (2002-05-01). "A stable diaminocarbene". J. Am. Chem. Soc. 117 (44): 11027–11028. doi:10.1021/ja00149a034.
- ^ Fillman KL, Przyojski JA, Al-Afyouni MH, Tonzetich ZJ, Neidig ML (February 2015). "N-heterocyclic carbene complexes". Chemical Science. 6 (2): 1178–1188. doi:10.1039/c4sc02791d. PMC 4302958. PMID 25621143.
- ^ Przyojski JA, Veggeberg KP, Arman HD, Tonzetich ZJ (2015-09-08). "Mechanistic Studies of Catalytic Carbon–Carbon Cross-Coupling by Well-Defined Iron NHC Complexes". ACS Catalysis. 5 (10): 5938–5946. doi:10.1021/acscatal.5b01445.
- ^ Przyojski JA, Arman HD, Tonzetich ZJ (2012-12-18). "NHC Complexes of Cobalt(II) Relevant to Catalytic C–C Coupling Reactions". Organometallics. 32 (3): 723–732. doi:10.1021/om3010756.
- ^ Herrmann, W. A. (1982). "The methylene bridge: A Challenge to Synthetic, Mechanistic and Structural Organometallic Chemistry". Pure and Applied Chemistry. 54: 65–82. doi:10.1351/pac198254010065.
- ^ Mol, J (2004-04-13). "Industrial applications of olefin metathesis". Journal of Molecular Catalysis A: Chemical. 213 (1): 39–45. doi:10.1016/j.molcata.2003.10.049. ISSN 1381-1169.
- ^ Davies, Huw M. L.; Morton, Daniel (2011-03-21). "Guiding principles for site selective and stereoselective intermolecular C–H functionalization by donor/acceptor rhodium carbenes". Chemical Society Reviews. 40 (4): 1857–1869. doi:10.1039/C0CS00217H. ISSN 1460-4744.
- ^ Denmark, Scott E., ed. (2004-04-30). Organic Reactions (1 ed.). Wiley. doi:10.1002/0471264180.or070.02. ISBN 978-0-471-26418-7.
- ^ a b Hahn, F. Ekkehardt; Jahnke, Mareike C. (2008-04-14). "Heterocyclic Carbenes: Synthesis and Coordination Chemistry". Angewandte Chemie International Edition. 47 (17): 3122–3172. doi:10.1002/anie.200703883. ISSN 1433-7851.
- ^ Fischer, E. O.; Maasböl, A. (1964-08). "On the Existence of a Tungsten Carbonyl Carbene Complex". Angewandte Chemie International Edition in English. 3 (8): 580–581. doi:10.1002/anie.196405801. ISSN 0570-0833.
{{cite journal}}: Check date values in:|date=(help) - ^ Wanzlick, H.‐W.; Schönherr, H.‐J. (1968-02). "Direct Synthesis of a Mercury Salt‐Carbene Complex". Angewandte Chemie International Edition in English. 7 (2): 141–142. doi:10.1002/anie.196801412. ISSN 0570-0833.
{{cite journal}}: Check date values in:|date=(help) - ^ Öfele, K. (1968-06). "1,3-Dimethyl-4-imidazolinyliden-(2)-pentacarbonylchrom ein neuer übergangsmetall-carben-komplex". Journal of Organometallic Chemistry. 12 (3): P42–P43. doi:10.1016/s0022-328x(00)88691-x. ISSN 0022-328X.
{{cite journal}}: Check date values in:|date=(help)
