User:Mr. Ibrahem/Testosterone (medication)
 | |
 | |
| Clinical data | |
|---|---|
| Pronunciation | /tɛˈstɒstəroʊn/ teh-STOS-tə-rohn[1] |
| Trade names | AndroGel, Testim, TestoGel, others |
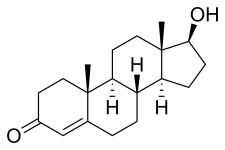
| Other names | Androst-4-en-17β-ol-3-one |
| AHFS/Drugs.com | Monograph |
| MedlinePlus | a619028 |
| License data |
|
| Pregnancy category |
|
| Routes of administration | By mouth, buccal, sublingual, intranasal, transdermal, vaginal, rectal, intramuscular or subcutaneous injection, subcutaneous implant |
| Drug class | Androgen, anabolic steroid |
| Legal status | |
| Legal status |
|
| Pharmacokinetic data | |
| Bioavailability | By mouth: very low (due to extensive first pass metabolism) |
| Protein binding | 97.0–99.5% (to SHBG and albumin)[3] |
| Metabolism | Liver (mainly reduction and conjugation) |
| Elimination half-life | 2–4 hours[citation needed] |
| Excretion | Urine (90%), feces (6%) |
| Identifiers | |
| |
| Chemical and physical data | |
| Formula | C19H28O2 |
| Molar mass | 288.431 g·mol−1 |
| 3D model (JSmol) | |
| Specific rotation | +110.2° |
| Melting point | 155 °C (311 °F) |
| |
| |
| (verify) | |
Testosterone (T) is a medication and naturally occurring steroid hormone.[5] It is used to treat male hypogonadism, gender dysphoria, and certain types of breast cancer.[5][6] It may also be used to increase athletic ability in the form of doping.[5] It is unclear if the use of testosterone for low levels due to aging is beneficial or harmful.[7] Testosterone can be used as a gel or patch that is applied to the skin, injection into a muscle, tablet that is placed in the cheek, or tablet that is taken by mouth.[5]
Common side effects of testosterone include acne, swelling, and breast enlargement in men.[5] Serious side effects may include liver toxicity, heart disease, and behavioral changes.[5] Women and children who are exposed may develop masculinization.[5] It is recommended that individuals with prostate cancer not use the medication.[5] It can cause harm to the baby if used during pregnancy or breastfeeding.[5] Testosterone is in the androgen family of medications.[5]
Testosterone was first isolated in 1935, and approved for medical use in 1939.[8][9] Rates of use have increased three times in the United States between 2001 and 2011.[10] It is on the World Health Organization's List of Essential Medicines.[11] It is available as a generic medication.[5] The price depends on the dose and form of the product.[12] In 2017, it was the 132nd most commonly prescribed medication in the United States, with more than five million prescriptions.[13][14]
References
[edit]- ^ Testosterone Archived June 13, 2018, at the Wayback Machine. Oxford Dictionaries.
- ^ a b "Testosterone Use During Pregnancy". Drugs.com. August 20, 2019. Archived from the original on February 1, 2014. Retrieved January 8, 2020.
- ^ Melmed, Shlomo; Polonsky, Kenneth S.; Larsen, P. Reed; Kronenberg, Henry M. (November 11, 2015). Williams Textbook of Endocrinology. Elsevier Health Sciences. pp. 709, 711, 765. ISBN 978-0-323-34157-8. Archived from the original on April 14, 2019. Retrieved November 18, 2016.
- ^ Cite error: The named reference
impwas invoked but never defined (see the help page). - ^ a b c d e f g h i j k "Testosterone". Drugs.com. American Society of Health-System Pharmacists. December 4, 2015. Archived from the original on August 20, 2016. Retrieved September 3, 2016.
- ^ "List of Gender Dysphoria Medications (6 Compared)". Drugs.com. Archived from the original on April 26, 2020. Retrieved May 6, 2020.
- ^ Staff (March 3, 2015). "Testosterone Products: Drug Safety Communication – FDA Cautions About Using Testosterone Products for Low Testosterone Due to Aging; Requires Labeling Change to Inform of Possible Increased Risk of Heart Attack And Stroke". FDA. Archived from the original on March 5, 2015. Retrieved March 5, 2015.
- ^ Taylor, William N (2002). Anabolic Steroids and the Athlete (2 ed.). McFarland. p. 180. ISBN 978-0-7864-1128-3. Archived from the original on September 14, 2016.
- ^ Fischer, Jnos; Ganellin, C. Robin (2006). Analogue-based Drug Discovery. John Wiley & Sons. p. 481. ISBN 9783527607495. Archived from the original on March 1, 2019. Retrieved March 1, 2019.
- ^ Desroches B, Kohn TP, Welliver C, Pastuszak AW (April 2016). "Testosterone therapy in the new era of Food and Drug Administration oversight". Translational Andrology and Urology. 5 (2): 207–12. doi:10.21037/tau.2016.03.13. PMC 4837303. PMID 27141448.
{{cite journal}}: CS1 maint: unflagged free DOI (link) - ^ World Health Organization (2019). World Health Organization model list of essential medicines: 21st list 2019. Geneva: World Health Organization. hdl:10665/325771. WHO/MVP/EMP/IAU/2019.06. License: CC BY-NC-SA 3.0 IGO.
- ^ Hamilton, Richart (2015). Tarascon Pocket Pharmacopoeia 2015 Deluxe Lab-Coat Edition. Jones & Bartlett Learning. p. 197. ISBN 978-1-284-05756-0.
- ^ "The Top 300 of 2020". ClinCalc. Archived from the original on March 18, 2020. Retrieved April 11, 2020.
- ^ "Testosterone - Drug Usage Statistics". ClinCalc. Archived from the original on July 8, 2020. Retrieved April 11, 2020.
