User:Mlunde330
Hi world. I am using this page to work on editing my page for Bio73
Lofexidine is an alpha2-adrenergic receptor agonist, historically used as a short-acting anti-hypertensive, but more commonly used to alleviate physical symptoms of heroin and opiate withdrawal.
Indication
[edit]In the United Kingdom, the hydrochloride form, lofexidine HCl, has been licensed and sold since 1992 for opiate withdrawal relief in tablet form as BritLofex by Britannia Pharmacuetical. BritLofex is only available by prescription. Lofexidine is also commonly used in conjunction with the opioid receptor agonist naltrexone in rapid detoxification cases. When these two drugs are paired, naltrexone is administered to relieve craving of the opioid, while lofexidine is given to relive physical withdrawal symptoms including chills, sweating, stomach cramps, muscle pain, and runny nose. As opiate withdrawal relief, lofexidine works to restore natural levels of norepinephrine and endorphins to pre-opiate addiction levels[1].
Clinical Similarities to Methadone
[edit]While clinically equivalent to methadone, lofexidine is not an opiate, as methadone is, and therefore does not have the addictive and dependent qualities of methadone. Indeed, one suggested use for lofexidine is to ease withdrawal symptoms of methadone dependence. Additionally, a significantly smaller dose is required, as the maximum recommended dose of BritLofex is 2.4 mg/day (0.2 mg/tablet) while a minimum effective methadone administration is about 40 mg/dose, and can increase to ~120 mg/dose. While abstaining from opiates and taking lofexidine, effective detoxification can succeed in as little as 3 days[2] , although the standard duration of detoxification using lofexidine is 10 days[3]. The [[LD50]] of lofexidine is 77 mg/kg. Lofexidine is not currently available in the United States. Britannia Pharmaceuticals has licensed lofexidine to be sold by US World Meds for sale in North America [4], and clinical trials are currently underway to secure approval for sale in the United States by the FDA[5].
An additional benefit of lofexidine treatment is that it is given as part of an outpatient, or ambulatory regimen, and can be completed without a hospital stay. This reduces costs for both healthcare provider and patient, and keeps specialist hospital beds free for particularly difficult withdrawal cases.
Structural Similarities to Clonidine
[edit]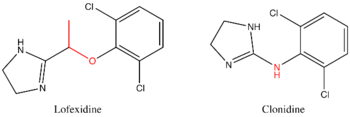
Lofexidine is structurally analogous to clonidine, another alpha2-adrenergic receptor agonist used for treatment of opioid withdrawal symptoms. A comparison of the two structures is shown at right. Both contain an imidazole ring and a 2,6-dichlorinated phenyl ring. The differences in structure are shown in red, while the similarities are in black. In addition to the structural differences, administration of lofexidine in heroin addicts has been shown to be more effective for a longer duration, with fewer withdrawal symptoms than clonidine even after one day[2]. Additionally, clonidine has been shown to significantly lower blood pressure. Therefore, although similar to lofexidine, clonidine is most frequently prescribed to treat high-blood pressure.
Other Clinical Uses
[edit]The possibility of using lofexidine to treat alcohol addiction withdrawal symptoms has been investigated, and has not yet been shown to be an effective treatment[6].
Additionally, the possibility of lofexidine to treat ADHD symptoms in children has been investigated[7]. Although this subject has only a small body of research, when a small dose of lofexidine is given (0.4 mg, 3x daily), ADHD severity significantly decreased. Similar studies have shown reduction of tics in children with the small dosage[7].
- Drowsiness
- Dry mouth
- Dry Nose
- Hypotension
- Dizziness
References
[edit]- ^ http://www.lofexidine.co.uk/how.htm
- ^ a b G. Gerra, et al, Lofexidine versus clonidine in rapid opiate detoxification, Journal of Substance Abuse TreatmentVolume 21, Issue 1, , July 2001, Pages 11-17.
- ^ J. Bearn, M. Gossop, J. Strang, Drug Alcohol Depend. 43, 87 (1996)
- ^ http://www.britannia-pharm.co.uk/
- ^ http://www.usworldmeds.com/lofexidine.htm
- ^ Keaney F, Strang J, Gossop M, Marshall EJ, Farrell M, Welch S, Hahn B, Gonzalez A. A double-blind randomized placebo-controlled trial of lofexidine in alcohol withdrawal: lofexidine is not a useful adjunct to chlordiazepoxide. Alcohol Alcohol (2001) 36:426–30.
- ^ a b Niederhofer H, Staffen W, Mair A: A placebo-controlled study of lofexidine in the treatment of children with tic disorders and attention deficit hyperactivity disorder. J Psychopharmacol 2003, 17:113-119.
- ^ http://www.lofexidine.co.uk/leaflets/bfl-pil-v4_07-2006.pdf
See also
[edit]
