User:Mevans86/Electrophilic amination
Electrophilic amination involves the formation of a carbon-nitrogen bond through the reaction of a nucleophilic carbanion with an electrophilic source of nitrogen[1].
Introduction
[edit]Electrophilic amination reactions can be classified as either additions or substitutions. Although the resulting product is not always an amine, these reactions are united by the formation of a carbon-nitrogen bond and the use of an electrophilic aminating agent. A wide variety of electrophiles have been used; for substitutions, these are most commonly amines substituted with electron-withdrawing groups: chloramines, hydroxylamines, hydrazines, and oxaziridines, for instance. Addition reactions have employed imines, oximes, azides, azo compounds, and others.
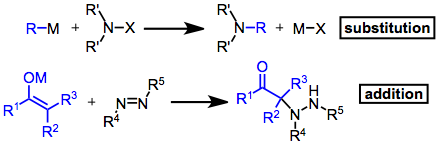
Mechanism and Stereochemistry
[edit]Prevailing Mechanisms
[edit]Nitrenoids can be generated from O-alkylhydroxylamines via deprotonation or from O-alkyloximes via nucleophilic addition. These intermediates react with carbanions to give substituted amines. Other electron-deficient, sp3 amination reagents react by similar mechanisms to give substitution products.

For oxaziridines, SN2 attack on nitrogen takes place. For some substrates (α-cyano ketones, for example), the resulting alkoxide is non-innocent, reacting to produce unexpected products. N-acyl-substituted oxaziridines give amides, rather than amines, as products.

Additions across pi bonds appear to proceed by typical nucleophilic addition pathways in most cases. One important reaction that has been well studied is that of the addition of carbanions to azides, providing either triazines or amines. When triazenes are isolated two isomers are possible, and in some cases the isomer actually isolated has not been ascertained with certainty. Acid treatment of aryl triazenes gives aromatic amines.

Enantioselective Variants
[edit]The most synthetically useful aminations of enolate anions incorporate N-acyloxazolidinone substrates. The chiral auxilliaries on these compounds are easily removed after hydrazine formation (with azo compounds) or azidation (with trisyl azide). Azidation using the latter reagent is more efficient than bromination followed by nucleophilic substitution by the azide anion.

Scope and Limitations
[edit]Coming soon!
Synthetic Applications
[edit]Coming soon!
Comparison with Other Methods
[edit]Coming soon!
Experimental Conditions and Procedure
[edit]Typical Conditions
[edit]The wide variety of electrophilic aminating reagents precludes generalization of reaction conditions. Electrophilic nitrogen sources are, however, either toxic or explosive in general. Great care should be taken while handling these reagents. Many electrophilic nitrogen sources do not provide amines immediately, but a number of methods exist to generate the corresponding amines.
- Tosylamines: tributyl tin hydride
- Azo compounds: H2/Pd
- Triazenes: sodium borohydride
- Azides: H2/Pd, H2/Pt, lithium aluminum hydride, triphenylphosphine
Conversion to other nitrogen-containing functionality, including enamines, imines, and amides, is also possible.
Example Procedure
[edit]A freshly prepared solution of potassium tert-butoxide (31 mg, 0.28 mmol) in THF (2 mL) was added slowly to a solution of ethyl phenylacetate (41 mg; 0.25 mmol) in THF (3 mL) cooled to –78° and the mixture was stirred at –78° for 15 minutes. O-[Di(p-methoxyphenyl)]phosphinoylhydroxylamine (caution, related hydroxylamine derivatives are explosive) (81 mg, 0.28 mmol) was added as a solid in one portion, and the mixture was left to warm to room temperature and stirred overnight. Acetic anhydride (71 μL, 0.75 mmol) and triethylamine (210 μL, 1.5 mmol) were added and the mixture was stirred at room temperature for one hour. Et2O (20 mL) and saturated aqueous NH4Cl solution (30 mL) were added, and the aqueous layer was extracted with Et2O (2 x 30 mL). The dried (MgSO4) extracts were concentrated and the residue was purified by flash chromatography (1:1 EtOAc:cyclohexane) to give 37 mg (67%) of the title product as a colorless oil, Rf 0.20 (1:1 EtOAc:cyclohexane). No other data were reported.

References
[edit]- ^ Ciganek, E. Org. React. 2008, 72, 1-86.
