User:Manishearth/Bigcontribs
This page includes my major edits. The topmost ones are the most recent.
Scripts
[edit]More information on each script is at User:Manishearth/Scripts
User:Manishearth/sidebartoggle.js (Toggles visibility of the sidebar, with a save preferences option)
User:Manishearth/sidebartranslinks.js (Allows user to expand scope of research by one-clock translations of the same page on other wikis)
User:Manishearth/orphantabs.js (Doc at User:Manishearth/OrphanTabs)
User:Manishearth/editns.js (Allows use of "Edit:" namespace)
User:Manishearth/patroltab.js(Gives a "mark as patrol" tab)
Rather large, please see it User:Manishearth/Bigcontribs/deva-conjuncts
- Identify the parent hydrocarbon chain (The longest continuous chain of carbon atoms)
- Identify the functional group, if any (If more than one, use the one with highest precedence)
- Identify the position of the functional group.
- Number the carbon atoms in the parent chain. The functional group should have the lower number (as there are two ways of numbering—right to left and left to right). The number is written before the name of the functional group suffix (such as -ol, -one, -al, etc.). If the group is a terminal-only group, that can only be at the end of a chain (such as the carboxylic acid and aldehyde groups), it need not be numbered.
NOTE: If there are no functional groups, number in both directions, find the numbers of the side-chains(the carbon chains that are not in the parent chain) in both directions. The end result should be such that the placements of side chains have a lower number; for example, 2,2,5-Trimethylhexane is prefered over 2,5,5-Trimethylhexane.
- Identify the side-chains and number them.
- If there are more than one of the same type of side-chain, add the prefix (di-,tri, etc.) before it. The numbers for that type of sidechain will be grouped in ascending order and written before the name of the side-chain. If there are two side-chains on the same carbon atom, the number will be written twice.
- Different side-chains will be grouped in alphabetical order (The prefixes di-,tri-,etc. are not taken into consideration for grouping alphabetically. For example, ethyl comes before dimethyl, as the "e" in "ethyl" is alphabetically precedent to the "m" in "methyl". The "di" is not considered).
- Identify the remaining functional groups, if any, and group their numbers and ion names (Such as hydroxy for -OH, oxy for =O , oxyalkane for O-R, etc.) alphabetically with the carbon chains using the same method.
- Identify double/triple bonds. Number them with the number of the carbon atom before them. For example a double bond between carbon atoms 3 and four is numbered as 3-ene. many double bonds are named with a prefix(di-, tri-, etc.)
- Arrange everything like this: Group of side chains and secondary functional groups + prefix of parent hydrocarbon chain (eth, meth) + double/triple bonds + primary functional group
- Add punctuation:
- Put commas between numbers (2 5 5 becomes 2,5,5)
- Put hyphens between numbers and letters (2 5 5 trimethylhexane becomes 2,5,5-tremethylhexane)
- Successive words are merged into one word (trimethyl hexane becomes trimethylhexane)
NOTE: IUPAC uses one-word names throughout. This is why all parts are connected.
The finalized name should look like this:
#,#-di<side chain>-#-<side chain>-#-<secondary functional group><parent chain suffix><If all bonds are single bonds, use "ane">-#-<double bonds>-#-<triple bonds>-#-<primary functional group>
NOTE: # is used for a number, and all fields other than the parent chain and bonds are optional. The side chains and secondary functional groups are arranged alphabetically.
Example:
Here is a sample molecule with the parent carbons numbered:
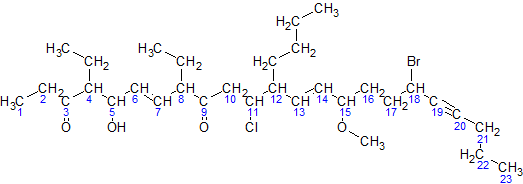
For simplicity, here is an image of the same molecule, where the carbons in the parent chain are removed:

Now, we go by the steps:
- The parent hydrocarbon chain has 23 carbons. It is called tricos-.
- The functional groups with the highest precedence are the two ketone groups.
- The groups are on carbon atoms 3 & 9. As there are two, we write 3,9-dione.
- The numbering of the molecule is based on the ketone groups. when numbering from left to right, the ketone groups get numbered 3 and 9.when numbering from right to left, the ketone groups get numbered 15 and 21. The sum of 3 & 9 (12) is less than the sum of 15 & 21 (36). Therefore, the numbering s done left to right, and the ketones are numbered 3 & 9.
- The side chains are: an ethyl- at carbon 4, an ethyl- at carbon 8, and a butyl- at carbon 12.
NOTE:The -O-CH3 at carbon atom 15 is not a side chain, but it is a methoxy functional group- There are two ethyl- groups, so they are combined to create, 4,8-diethyl.
- The side chains shall be grouped like this: 12-butyl-4,8-diethyl
- The secondary functional groups are: a hydroxy- at carbon 5, a chloro- at carbon 11, a methoxy- at carbon 15, and a bromo- at carbon 18. Grouped with the side chains, we get 18-bromo-12-butyl-11-chloro-4,8-diethyl-5-hydroxy-15-methoxy
- There are two double bonds: one between carbons 6 & 7, and one between carbons 13 & 14. They will be called 6,13-diene. There is one triple bond between carbon atoms 19 & 20. It will be called 19-yne
- The arrangement(with punctuation) is: 18-bromo-12-butyl-11-chloro-4,8-diethyl-5-hydroxy-15-methoxytricos-6,13-diene-19-yne-3,9-dione
Alk is the prefix of the group (Meth, Eth, Prop, etc.)
| Devanagari | अ | आ | इ | ई | उ | ऊ | ए | ऐ | ओ | औ | अं | अः | ऋ |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Transliterated | a | ā | i | ī | u | ū | e | ai | o | au | aṃ | aḥ | ṛ |
| IPA | /ə/ | /a/ | /i/ | /u/ | /e/ | /əi/ | /o/ | /əu/ | /əⁿ/ | /əh/ | /ru/ | ||
| Pronunciation | |||||||||||||
| क | ख | ग | घ | ङ |
|---|---|---|---|---|
| ka /kə/ |
kha /kʰə/ |
ga /ɡə/ |
gha /ɡʰə/ |
ṅa /ŋə/ |
| च | छ | ज | झ | ञ |
| ca /cɕə/ or /ʦə/ |
cha /cɕʰə/ or /ʦʰə/ |
ja /ɟʝə/ or /zə/ |
jha /ɟʝʰə/ or /zʰə/ |
ña /ɲə/ |
| ट | ठ | ड | ढ | ण |
| ṭa /ʈə/ |
ṭha /ʈʰə/ |
ḍa /ɖə/ |
ḍha /ɖʰə/ |
ṇa /ɳə/ |
| त | थ | द | ध | न |
| ta /t̪ə/ |
tha /t̪ʰə/ |
da /d̪ə/ |
dha /d̪ʰə/ |
na /n̪ə/ |
| प | फ | ब | भ | म |
| pa /pə/ |
pha /pʰə/ or /fə/ |
ba /bə/ |
bha /bʰə/ |
ma /mə/ |
| य | र | ऱ | ल | व |
| ya /jə/ |
ra /rə/ |
ṟa /?/ |
la /lə/ |
va /və/ or /wə/ |
| श | ष | स | ह | |
| śa /ʃə/ |
ṣa /ʃə/ |
sa /sə/ |
ha /hə/ |
|
| ळ | क्ष | ज्ञ | ||
| ḷa /ɭə/ |
kṣa /kʃə/ |
jña /ɟʝɲə/ |