User:MUBISC-AR/sandbox
Carol A Barnes, Ph.D.
[edit]Education:
[edit]



Carol Barnes received a Bachelors of Arts in Psychology with honors at the University of California, riverside, in 1971. She continued her education at Carleton University located in Ottawa Ontario, Canada, earning her Masters of Arts degree in Psychology in 1972.During her studies in pursuing a PhD in Psychology, Barnes took a temporary position as a postdoctoral researcher researching in neuropsychology and neurophysiology in the department of psychology at Dalhousie University, at the institute of neurophysiology of University of Osloas well as in Cerebral function at University College of London. In 1977 she graduated with Cum Laude in psychology at Carleton University.
Career:
[edit]Barnes currently holds the positions of director for two organizations: Evelyn F McKnight Brain Institute and Neural Systems-Memory and Aging. She is also the Endowed Chair of the Evelyn F McKnight Brain Institute for Learning-Memory Aging. Today, she is a regents professor at the University of Arizona in Tucson, Arizona, of the BIO5 Institute, funded by the Technology and Research Fund (TRIF)[1]. At the BIO5 institute she works with students addressing Alzheimer's disease and other age-related brain diseases. Barnes also teaches cancer biology, neuroscience, psychology, and physiological sciences.
Research:
[edit]Barnes is involved with the neuroscience research community on a national and local level. Barnes research spans through 4 decades aiming to better understand the aging of the brain in relation to cognitive diseases. Her research is conducted by utilizing animal models such as primates and rats to explore and understand how memory is impacted through the normal aging process of the brain through means of neurobiological mechanisms that are involved. The use of animal models in Barnes research is translated into treatments used for abnormal functioning of the brain aimed at prolonging the cognitive lifespan of older individuals. The nature of Barnes research entails the use of behavioral, anatomical, electrophysiological and molecular techniques to further examine the implications of normal aging impacting brain functioning.[2] Most areas of her research involves examining and observing the hippocampus structure in the brains of rats and monkeys, observing and recording synaptic transmission in cell to cell signaling, and genomic sequencing.
To study spatial learning and memory, Carol created her own maze to test whether rodents, specifically mice, could remember the location of an escape box on a platform. This test has become a standard method of memory testing in laboratories. Designed in 1979, Carol’s maze was an alternative to study memory without the use of external reward or punishment, while also reducing the stress put on the animal throughout the process.

The original study consisted of a platform (122cm diameter) raised 91 cm above the floor with 18 circular holes (9.5cm diameters) spaced evenly around the perimeter. Under one of the holes is a black escape box, or any dark color in contrast to the color of the platform; the rest of the holes lead to false escape boxes. The study consisted of 3 variations of the experiment. First, the mouse simply had to find the escape chamber that was placed under one of the holes. Second, the escape chamber was moved to a hole rotated 120-140 degrees from the original hole; the original hole was also covered. Third, the same method as the second variation was used except the original hole was not covered. Barnes and her team found that overall, male mice performed better in all variations of the experiment. Further, they found younger mice also performed better in all variations, while older mice demonstrated notable difficulties in variations 2 and 3. This showed aging impairment in spatial memory.[3]
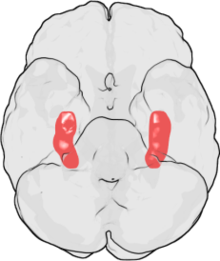
Spatial memory corresponds to the functioning of the hippocampus, while route learning involves the striatal systems. Overall, rodents of various ages learned T-mazes within the same number of trials, but the strategies used to learn and remember mazes differed with age. Young rats predominately utilized “place” strategies which rely upon hippocampal functioning, while older rats relied upon “response” strategies. Using MRI imaging techniques, Barnes and her team were able to see that in normally aged brains of rodents, the size of the hippocampus did not change while the volume of cortical grey matter did. This animal model allowed an understanding of what the brain looks like during normal human functioning to compare it to brains that have been degenerated due to Alzheimer’s disease (rodents do not exhibit AD). These findings demonstrate that while during normal aging the hippocampus remains the same size, its function may decrease in comparison to functions of other areas of the brain.[4]
Looking deeper into the tissue affected, single-cell imaging showed there were 3 main types of cells that make up the hippocampus. When observing cell activity and the number of cells of the hippocampus of rats, it was found that the CA1 and CA3 pyramidal cells continued to be active and of the same volume. However, the number of granule cells of the dentate gyrus continuously decreased with age; the function of these cells also declined leading Carol and her team to conclude these gyrus cells are the weak link of the hippocampal circuit involved in memory.[5]
A few of Barnes’s latest research publication was published in 2017 are: “Attentional updating and monitoring and affective shifting are impacted independently by aging in the macaque monkeys”, “Evidence for an evolutionarily conserved memory coding scheme in the mammalian hippocampus”, and “The aging navigational system”.
Primate Research:
[edit]
In addition to work on rodent models, Dr. Barnes has helped advance the field of normative aging research using nonhuman primate models, specifically macaque monkeys. Primarily, Barnes’ early work in macaques helped to tie together neurobiological data collected from rodents and functional imaging data from older humans. In the paper “Memory impairment in aged primates is associated with region-specific network dysfunction” Barnes and her team showed that older monkeys had significant impairment on object recognition than younger monkeys. In addition, the older monkeys displayed a lower density of inhibitory somatostatin positive interneurons in the CA3 sub-region of the hippocampus. These interneurons are responsible for regulating the activity of excitatory neurons in the hippocampus. With less interneuron density, the baseline firing rate of CA3 excitatory neurons was elevated, which resembles the increased hippocampal activity shown in imaging studies of older human adults. Both a decrease in interneuron density and increase baseline firing rates in the hippocampus have been associated with poor cognition.[6]
Furthermore, in the paper “Evidence for an evolutionarily conserved memory coding scheme in the mammalian hippocampus” Dr. Barnes and her team found evidence that mammals require the same quantity of neurons in the hippocampus to encode memory of a single experience. This finding means that the studied rodents, nonhuman primates, and humans all use a stable amount of neurons to encode a similar virtual experience. However, since all three subjects have different sized hippocampi, the proportion of neurons used for the encoding of experience differ. Rodents have the smallest hippocampus and thus use 40% of their hippocampus neurons for encoding, nonhuman primates have larger hippocampi and use 4%, and finally humans have the largest hippocampi and use an estimated 2.5% for experiential encoding.[7]
Dr. Barnes has also done research to see how executive function changes with normative aging. Executive functions are the higher order process humans take part in such as attention, decision making, impulse control, and emotional control. Once again studying macaques, Barnes and her team focused on two aspects of executive function, attentional monitoring and updating as well as set shifting. Attentional monitoring and updating are when the rules of a given situation change requiring a corresponding change in behavioral responses. For example, when two options are presented one is the correct initial choice. When the correct choice becomes the second option instead, attentional monitoring and updating help correct for the rule change and alter behavior. The change in behavior is mediated through a process of trial and error which helps associate desired outcomes with certain behaviors. Barnes and her team found that older monkeys needed a greater number of trials to accurately account for a rule change. Meaning, the executive system behind attentional monitoring and updating is impaired with aging. Secondly, set shifting is the ability to unconsciously shift attention between tasks while maintaining accuracy. In her research, Barnes presented macaque monkeys with an object recognition test of previously learned objects. She then presented interfering objects which required shifts between the object choice and evaluation of novel objects. Her results showed that older monkeys performed better on object recognition with interference than younger monkeys. Thus set shifting abilities seem to be maintained if not enhanced with aging. The most important discovery out of these studies, however, is that the two aspects of executive function, monitoring and updating and set shifting, were shown to be independent systems that are affected differently with age.[8]
A final research contribution of Carol A Barnes involves the study of spatial networks and spatial memories in aging macaques. Barnes and her team studied brain activity in four different conditions for movement: cages, sitting, walking on a treadmill, and freely walking around space. The study found that younger macaques have distinct spatial networks for all four distinct conditions. However, older monkeys displayed less differentiated activity of spatial networks. Meaning, all conditions evoked activation of the same undifferentiated networks. This finding suggests dynamic network changes as a possible explanation for spatial cognition deficits. In other words, the spatial processing networks become less precise with age and may contribute to spatial memory loss or confusion.[9]
Family:
[edit]Barnes interest of normal aging in the brain stems from her grandfather when she and her family noticed that his memory was declining.[10] Furthermore, Barnes is included in the Neuroscience Academic Family Tree . Listed there are her mentors and mentees.
Supporting Women and the Underprivileged in Neuroscience:
[edit]Carol A Barnes has been lauded by her peers and the public for her outstanding work in promoting opportunities for women and the underprivileged in neuroscience. In 2010, she received the Mika Salpeter Lifetime Achievement Award[10] which “recognizes individuals with outstanding career achievements in neuroscience who have also actively promoted the professional advancement of women in neuroscience.”[11]
Furthermore she is an active participant in the NIH Disadvantaged High School Student Research Program, Minority Access to Research Careers, and the McNair Achievement Program. Finally, in 2013 Barnes gave a keynote address at the Celebration of Women in Neuroscience entitled “The Evolving Face of Neuroscience: Role of Women and Globalization.”[10]
- National science Foundation Summer Research Fellowship, 1969
- This fellowship funds students or identity programs that are focused on education developments
- Ontario Graduate Fellowship, 1972-74
- For students pursuing their graduate studies at Master’s and doctoral levels. This is a merit-based scholarship for students a part of the Ontario Graduate program.
- NIH National Research Service Award, 1979-81
- For Postdoctoral students gaining research and clinical training in both MD/PhD or other dual-doctoral degree training program
- NATO Postdoctoral Fellowship in Science 1981-82
- Research Career Development Award, NIH, 1984-89[13]
- ADAMHA Research Scientist Development Award, National Institute of Mental Health, 1989-94
- ADAMHA Research Scientist Development Award, National Institute of Mental Health 1994-99
- Foreign Member, The Royal Norwegian society of Sciences and Letter (Natural Sciences), 2004-present
- MERIT Award, National Advisory Council on Aging, 2004-14
- Regents Professor, University of Arizona, 2006-present[14]
- Endowed Chair: Evelyn F. McKnight Chair Learning and Memory in Aging, 2006-present
- Fellow, American Association for the Advancement of Science, 2007-present
- Fellows are elected annually by the AAAS council. Fellows are recognized for their contributions to the advancement of science or its application are scientifically or socially distinguished.[15]
- 2009 APA Division 6 D.B. Marquis Behavioral Neuroscience Award, 2010
- 2010 APA Division 6 D.B. Marquis Behavioral Neuroscience Award for Behavioral Neuroscience
- Mika Salpeter Lifetime Achievement Award. 2010[10]
- Galileo Fellow, College of Science, University of Arizona, 2011-present
- Ralph W Gerard Prize in Neuroscience (2013)
- APA Award for Distinguished Scientific Contributions (2014)
- Barnes received this award as her role as director of the McKnight Brain Institute, the role in which she currently holds. This award is considered to be one of the highest awards of the Society for Neuroscience.
- Quad-L Award (2017)
- Barnes being one of the 7 people (including herself) who holds this award. This award is for distinguished contemporary scientists actively involved in research in regard to learning, memory or cognition.[16]
- MOCA Local Genius Award, 2017
- Elected to National Academy of Science, 2018
- This award is one of the highest honors. Barnes received this award for her distinguished and continuation of her original research.
 | This is a user sandbox of MUBISC-AR. You can use it for testing or practicing edits. This is not the sandbox where you should draft your assigned article for a dashboard.wikiedu.org course. To find the right sandbox for your assignment, visit your Dashboard course page and follow the Sandbox Draft link for your assigned article in the My Articles section. |
- ^ "Why BIO5 | BIO5 Institute". bio5.org. Retrieved 2020-03-28.
- ^ "Carol Barnes | Department of Psychology". psychology.arizona.edu. Retrieved 2020-03-28.
- ^ Gawel, Kinga; Gibula, Ewa; Marszalek-Grabska, Marta; Filarowska, Joanna; Kotlinska, Jolanta H. (2019-01-01). "Assessment of spatial learning and memory in the Barnes maze task in rodents—methodological consideration". Naunyn-Schmiedeberg's Archives of Pharmacology. 392 (1): 1–18. doi:10.1007/s00210-018-1589-y. ISSN 1432-1912. PMC 6311199. PMID 30470917.
{{cite journal}}: CS1 maint: PMC format (link) - ^ Samson, Rachel D.; Barnes, Carol A. (2013). "Impact of Aging Brain Circuits on Cognition". The European journal of neuroscience. 37 (12): 1903–1915. doi:10.1111/ejn.12183. ISSN 0953-816X. PMC 3694726. PMID 23773059.
- ^ Barnes, Carol A. (2011-10-03). "Secrets of aging: What does a normally aging brain look like?". F1000 Biology Reports. 3. doi:10.3410/B3-22. ISSN 1757-594X. PMC 3186042. PMID 22003369.
- ^ Gray, Daniel T.; Barnes, Carol A. (2019-12-26). "Experiments in macaque monkeys provide critical insights into age-associated changes in cognitive and sensory function". Proceedings of the National Academy of Sciences of the United States of America. 116 (52): 26247–26254. doi:10.1073/pnas.1902279116. ISSN 0027-8424. PMC 6936691. PMID 31871147.
- ^ Wixted, John T.; Goldinger, Stephen D.; Squire, Larry R.; Kuhn, Joel R.; Papesh, Megan H.; Smith, Kris A.; Treiman, David M.; Steinmetz, Peter N. (2018-01-30). "Coding of episodic memory in the human hippocampus". Proceedings of the National Academy of Sciences. 115 (5): 1093–1098. doi:10.1073/pnas.1716443115. ISSN 0027-8424. PMID 29339476.
- ^ Gray, Daniel T.; Smith, Anne C.; Burke, Sara N.; Gazzaley, Adam; Barnes, Carol A. (2017-03-30). "Attentional updating and monitoring and affective shifting are impacted independently by aging in macaque monkeys". Behavioural brain research. 322 (Pt B): 329–338. doi:10.1016/j.bbr.2016.06.056. ISSN 0166-4328. PMC 5493156. PMID 27368416.
- ^ Engle, James R.; Machado, Christopher J.; Permenter, Michele R.; Vogt, Julie A.; Maurer, Andrew P.; Bulleri, Alicia M.; Barnes, Carol A. (2016-11-30). "Network Patterns Associated with Navigation Behaviors Are Altered in Aged Nonhuman Primates". Journal of Neuroscience. 36 (48): 12217–12227. doi:10.1523/JNEUROSCI.4116-15.2016. ISSN 0270-6474. PMID 27903730.
- ^ a b c d Sherva, Kara (2015). "Carol Barnes: A Prominent Voice in the Neuroscience of Aging, and a Proponent of Women in Neuroscience". Sound Neuroscience: An Undergraduate Neuroscience Journal. 2.
- ^ "Awards". www.sfn.org. Retrieved 2020-03-28.
- ^ "Dr. Carol A. Barnes - Honors and Awards | Evelyn F. McKnight Brain Institute". embi.arizona.edu. Retrieved 2020-03-28.
- ^ "Research Career Development Awards | Research Training and Career Development". researchtraining.nih.gov. Retrieved 2020-03-28.
- ^ "Regents' Professors | UA Faculty Affairs". facultyaffairs.arizona.edu. Retrieved 2020-03-28.
- ^ "AAAS Honorary Fellows". American Association for the Advancement of Science. Retrieved 2020-03-28.
- ^ "Dr. Barnes receives the Quad-L Award | Evelyn F. McKnight Brain Institute". embi.arizona.edu. Retrieved 2020-03-28.
