User:LouisBB/OrgChem
My remarks on OrgChem Talkpage:
[edit][edit] Looking at the peer review Since I looked at this article last it has much improved, mainly the first part, but I think that the introductory sentence would be clearer broken into two.
1) Heeding the peer reviewer I agree, that there are several brief statements made here and there without explanation or even a link
Historic highlights last para last sentence promising = promising what? Historic highlights penultimate para too hard to follow Hydrocarbons and functional groups third para after ...unless etc = explain?/reference?/wipe? 2) Characteristics section still needs reorganising:
Because both characteristics and organic reactions depend on the same factors (molecular structure/atomic structure which are factors thoroughly discussed in other works on Organic chemistry but not YET by us) I would group them one after the other I would break up Characteristics to Chemical, and Physical, and detail under characteristics why and how atomic and molecular structure influences them I would explain as explained by Richard and Sally Daley (Ref 2.)why we pick on those few physical characteristics that we discuss here: because they give us clues on determining what compound we are looking at in the lab. I think it is important to at least mention the effect of the prevailing conditions on both the physical and chemical characteristics: We do not mention anywhere for instance that organic compounds, like inorganic ones are found in three states of aggregation (forgetting about the fourth) 3) Organic reactions
We ought to mention I) the main reaction types of the organo-chemical reactions and II) define the Named reactions and say where we can find a list for them All this is rather a lot still to make it really VG - I think. I shall do something in my sandbox (User:LouisBB/OrgChem), because it would be too much here. Feel free to criticise.
I admit, I am poor on references, but in a small village I have no library access. LouisBB 15:33, 29 March 2007 (UTC)
Retrieved from "http://en.wikipedia.org/wiki/Talk:Organic_chemistry"
- The article OrgChem is copied and transferred out of WP to work on; When I have something I shall bring it here to be criticised
Suggestion for reorganisation
[edit]If we brought 'Characteristics of organic substances' and 'Organic reactions' in front of 'Classificatio' some duplication (eg about multiple bonding ) could be avoided
A first attempt at reforming 'Characteristics' is attempted. Ready for harsh critics
Organic chemistry is a specific discipline within chemistry which involves the scientific study of the structure, properties, composition, reactions, and preparation (by synthesis or by other means) of chemical compounds consisting of primarily carbon and hydrogen, which may contain any number of other elements, including nitrogen, oxygen, halogens as well as phosphorus, silicon and sulfur.[1][2]
The original definition of organic chemistry came from the misperception that these compounds were always related to life processes, but it has been shown that this is not the case (See Historic highlights). Moreover, it is known that life also depends heavily on inorganic chemistry; for example, many enzymes rely on transition metals such as iron and copper; and materials such as shells, teeth and bones are part organic, part inorganic in composition. These are to be added to the metals mentioned above, HCl solution used in the digestion of food and the water, the main constituent of all living creatures, which are all subjects also of inorganic chemistry. Apart from elemental carbon, inorganic chemistry deals only with simple carbon compounds, with molecular structures which do not contain carbon to carbon connections (its oxides, acids, salts, carbides, and minerals). This does not mean that single-carbon organic compounds do not exist (viz. methane and its simple derivatives). Compounds that are related to life processes are dealt with in the branch of chemistry which is called biochemistry.
Because of their unique properties, multi-carbon compounds exhibit extremely large variety and the range of application of organic compounds is enormous. They form the basis of, or are important constituents of many products (paints, plastics, food, explosives, drugs, petrochemicals, and many others) and of course (apart from a very few exceptions) they form the basis of all life processes.
The different shapes and chemical reactivities of organic molecules provide an astonishing variety of functions, like those of enzyme catalysts in biochemical reactions of live systems. The autopropagating nature of these organic chemicals is what life is all about.
Because of the special properties of carbon, it is likely that life in other star systems would be carbon-based, in spite of speculations about the possibility of substituting silicon, which lies just below carbon in the periodic table.
Trends in organic chemistry include chiral synthesis, green chemistry, microwave chemistry, fullerenes and microwave spectroscopy.
Historical highlights
[edit]
At the beginning of the nineteenth century, chemists generally thought that compounds from living organisms were too complicated in structure to be capable of artificial synthesis from non-living things, and that a 'vital force' or vitalism conferred the characteristics of living beings on this form of matter. They named these compounds 'organic', and preferred to direct their investigations toward inorganic materials that seemed more promising.
Organic chemistry received a boost when it was realized that these compounds could be treated in ways similar to inorganic compounds and could be created in the laboratory by means other than 'vital force'. Around 1816 Michel Chevreul started a study of soaps made from various fats and alkali. He separated the different acids that, in combination with the alkali, produced the soap. Since these were all individual compounds, he demonstrated that it was possible to make a chemical change in various fats (which traditionally come from organic sources), producing new compounds, without 'vital force'.
The real event that has completely destroyed the myth of 'vitalism' occurred, however, when in 1828 Friedrich Wöhler first manufactured the organic chemical urea (carbamide), a constituent of urine, from the inorganic ammonium cyanate NH4OCN, in what is now called the Wöhler synthesis. Although Wöhler was, at this time as well as afterwards, cautious about claiming that he had thereby destroyed the theory of vital force, most have looked to this event as the turning point.
A great next step was when in 1856 William Henry Perkin, while trying to manufacture quinine, again accidentally came to manufacture the organic dye now called Perkin's mauve, which by generating a huge amount of money greatly increased interest in organic chemistry. Another step was the laboratory preparation of DDT by Othmer Zeidler in 1874, but the insecticide properties of this compound were not discovered until much later.
The crucial breakthrough for the theory of organic chemistry was the concept of chemical structure, developed independently and simultaneously by Friedrich August Kekule and Archibald Scott Couper in 1858. Both men suggested that tetravalent carbon atoms could link to each other to form a carbon skeleton, and that the detailed patterns of atomic bonding could be discerned by skillful interpretations of appropriate chemical reactions.
The history of organic chemistry continues with the discovery of petroleum and its separation into fractions according to boiling ranges. The conversion of different compound types or individual compounds by various chemical processes created the petroleum chemistry leading to the birth of the petrochemical industry, which successfully manufactured artificial rubbers, the various organic adhesives, the property-modifying petroleum additives, and plastics.
The pharmaceutical industry began in the last decade of the 19th century when acetylsalicylic acid (aspirin) manufacture was started in Germany by Bayer.
Total synthesis of complex natural compounds started with urea, increased in complexity to glucose and terpineol, and in 1907, total synthesis was commercialized the first time by Gustaf Komppa with camphor. Pharmaceutical benefits have been substantial, for example cholesterol-related compounds have opened ways to synthesis of complex human hormones and their modified derivatives. Since the start of the 20th century, complexity of total syntheses has been increasing, with examples such as lysergic acid and vitamin B12. Today's targets feature tens of stereogenic centers that must be synthesized correctly with asymmetric synthesis.
Biochemistry, the chemistry of living organisms, their structure and interactions in vitro and inside living systems, has only started in the 20th century, opening up a brand new chapter of organic chemistry with enormous scope.
Verification of the correctness of all statements, syntax etc, and loads of links are required here yet
Characteristics of organic substances
[edit]On the understanding, that both chemical and physical characteristics of organic compounds depend on the ambient conditions the other critical determining factor determining their proprties is their molecular structure.
The effects of the ambient conditions on the chemical characteristics and their reactivities are discussed under the subjects of chemical equilibria, chemical reaction kinetics, and thermodynamics whereas physical characteristics under physics, general chemistry and physical chemistry.
The effect of the molecular structure is related to the structure of the constituent atoms and therefore their electronconfigurations, which determine the chemical bonding of molecules.
Bonding, structure, and chemical characteristics
[edit]Bonding and structure
[edit]In the atomic structure it is the negatively charged electrons on the outer electronshell, farthest from the positively charged nucleus (their number and distance from the center) which determines the chemical characteristics of an element and the characteristics of a molelule depends on the configuration of their electron orbits.
The laws that govern the electron orbits and their types are now well known The octet principle explain the ‘force’ that impels an atom towards the attainment of the outer electronconfiguration of the chemicaly stable and inert noble gases.
In the ionic bond a loosely held electron of an outer shell is thus attracted by a neigbouring atom that requires it to make up an octet, which will then circle the host nucleus exclusively. Because of the electric charge transfer the donor atom becomes a positive ion, and the acceptor a negativ one and due to this extremely strong electrostatic attraction between them a rigid molecule (solid salts) is formed.
In the covalent bond there is no such strong attraction, and the electrical forces act in a much more subtle way as the compounding atoms only share electrons instead of giving them away. This leads to much more flexibility, creating compounds in which the electrons are shared between two nuclei or amongst several, when the paths electrons intertwine, which can happen in an extremely complex way; The great majority of the chemical bonds in organic compounds are of this type, which devoids them of the rigidity typical of the componds held together by ionic bond
The sharing of electronpairs between their atomic nuclei is the simplest case, demonstrated conveniently here by a Lewis diagram of methane pictorially presented. Please do not form the impression from such digrams that the real picture is two-dimensional, because this is not the case.
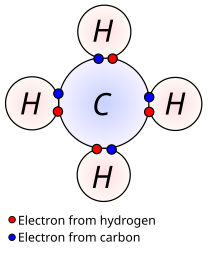
Imagining simplistically a pair of electrons whizzing around a couple of atomic nuclei at their farthest position from one another and knowing that the two nuclei are of unequal sizes it appears logical to suppose that in a time average the two electrons will be closer to one of the nuclei than to the other, which will make one end of the molecule more positive, the other more negative. Even if this should not be exactly the real case it is an easy way to imagine the formation this way of 'dipoles', oblong formations with a net, and opposing charge at its ends. The positively charged end of such a dipole will attract the negatively charged end of another which results in the type of bonding named a polar covalent bond.
I feel that expansion is needed here on Hydrogen bonding and Field Charge, and a Section on chemical characteristics depending on the presence or absence of functional groups etc Might be best to bring the Functional Groups section here
Organic compounds are generally covalently bonded. This allows for unique structures such as long carbon chains and rings. The reason carbon is excellent at forming unique structures and that there are so many carbon compounds is that carbon atoms form very stable covalent bonds with one another (catenation).
I feel that this para ought to be slightly modified to get rid of duplicate statements
Physical properties
[edit]A definition is needed for what we mean by that, reference to state of aggregation, and why we pick on the properties discussed here
In contrast to inorganic materials, organic compounds typically melt, boil, sublimate, or decompose below 300 °C. Neutral organic compounds tend to be less soluble in water compared to many inorganic salts, with the exception of certain compounds such as ionic organic compounds and low molecular weight alcohols and carboxylic acids where hydrogen bonding occurs.
Some of these statement do not hold true for all states of aggregation
We ought to define solubility/miscibility and refer the reader to further info on the complex nature of this subject
Organic compounds tend rather to dissolve in organic solvents which are either pure substances like ether or ethyl alcohol, or mixtures, such as the paraffinic solvents such as the various petroleum ethers and white spirits, or the range of pure or mixed aromatic solvents obtained from petroleum or tar fractions by physical separation or by chemical conversion. Solubility in the different solvents depends upon the solvent type and on the functional groups if present. Solutions are studied by the science of physical chemistry. Like inorganic salts, organic compounds may also form crystals.
See if there is anything in Classification' on the following subject and combine it here if there is any, to remove any duplication: to be inserted with Chemical properties
A unique property of carbon in organic compounds is that its valency does not always have to be taken up by atoms of other elements, and when it is not, a condition termed unsaturation results. In such cases we talk about carbon carbon double bonds or triple bonds. Double bonds alternating with single in a chain are called conjugated double bonds. An aromatic structure is a special case in which the conjugated chain is a closed ring.
Organic reactions
[edit]Organic reactions are chemical reactions involving organic compounds. While pure hydrocarbons undergo certain limited classes of reactions, many more reactions which organic compounds undergo are largely determined by functional groups. The general theory of these reactions involves careful analysis of such properties as the electron affinity of key atoms, bond strengths and steric hindrance. These issues can determine the relative stability of short-lived reactive intermediates, which usually directly determine the path of the reaction. An example of a common reaction is a substitution reaction written as:
- Nu− + C-X → C-Nu + X−
where X is some functional group and Nu is a nucleophile.
There are many important aspects of a specific reaction. Whether it will occur spontaneously or not is determined by the Gibbs free energy change of the reaction. The heat that is either produced or needed by the reaction is found from the total enthalpy change. Other concerns include whether side reactions occur from the same reaction conditions. Any side reactions which occur typically produce undesired compounds which may be anywhere from very easy or very difficult to separate from the desired compound.
Classification of organic substances
[edit]Description and nomenclature
[edit]Classification is not possible without having a full description of the individual compounds. In contrast with inorganic chemistry, in which describing a chemical compound could be achieved by simply enumerating the chemical symbols of the elements present in the compound together with the number of these elements in the molecule, in organic chemistry the relative arrangement of the atoms within a molecule has to be added for a full description.
One way of describing the molecule is by drawing its structural formula. Because of the complexity, this method has changed, becoming simplified over the years. The latest version is the line formula, which achieves simplicity without introducing ambiguity, while representing carbon and hydrogen by implication. The disadvantages which arise both from the fact that structural formulae cannot be described by words and that they are not easily printable does not arise when the structure is described by the organic nomenclature .
Because of the difficulty arising from the very large number and variety of organic compounds, chemists realized early on that the establishment of an internationally accepted system of naming organic compounds was of paramount importance. The Geneva Nomenclature was born in 1892 as a result of a number of international meetings on the subject.
It was also realized that as the family of organic compounds grew, the system would have to be expanded and modified. This task was ultimately taken on by the International Union on Pure and Applied Chemistry (IUPAC). Recognizing the fact that in the branch of biochemistry, the complexity of organic structures increases, the IUPAC organisation joined forces with the International Union of Biochemistry and Molecular Biology, IUBMB, to produce a list of joint recommendations on nomenclature.
Further on, as number and complexity grew, new recommendations were made within IUPAC for simplification. The first such recommendation was presented in 1951 when a cyclic benzene structure was named a cyclophane. Later recommendations extended the method to the simplification of other complex cyclic structures, including for instance heterocyclics as well, and named such structures phanes.
For ordinary communication, to spare a tedious description, the official IUPAC naming recommendations are not always followed in practice except when it is necessary to give a concise definition to a compound, or when the IUPAC name is simpler (viz. ethanol against ethyl alcohol). Otherwise the common or trivial name may be used, often derived from the source of the compound.
Classification
[edit]In summary: organic substances are classified by their molecular structural arrangement and by what other atoms are present with the chief (carbon) constituent in their makeup, whilst in a structural formula, hydrogen is implicitly assumed to occupy all free valences of an appropriate carbon atom, which remain after accounting for branching, other element(s) and/or multiple bonding.
Hydrocarbons and functional groups
[edit]
Classification normally starts with the hydrocarbons: compounds which contain only carbon and hydrogen. For sub-classes see below. Other elements present themselves in atomic configurations called functional groups which have decisive influence on the chemical and physical characteristics of the compound; thus those containing the same atomic formations have similar characteristics, which may be miscibility with water, acidity/alkalinity, chemical reactivity, oxidation resistance, or others. Some functional groups are also radicals, similar to those in inorganic chemistry, defined as polar atomic configurations which pass during chemical reactions from one chemical compound into another without change.
Some of the elements of the functional groups (O, S, N, halogens) may stand alone and the group name is not strictly appropriate, but because of their decisive effect on the way they modify the characteristics of the hydrocarbons in which they are present they are classed with the functional groups, and their specific effect on the properties lends excellent means for characterisation and classification.
Referring to the hydrocarbon types below, many, if not all of the functional groups which are typically present within aliphatic compounds are also represented within the aromatic and alicyclic group of compounds, unless they are dehydrated, which would lead to non-reacting co-optional groups.
Reference is made here again to the organic nomenclature, which shows an extensive (if not comprehensive) number of classes of compounds according to the presence of various functional groups, based on the IUPAC recommendations, but also some based on trivial names. Putting compounds in sub-classes becomes more difficult when more than one functional group is present.
Two overarching chain type categories exist: Open Chain aliphatic compounds and Closed Chain cyclic compounds. Those in which both open chain and cyclic parts are present are normally classed with the latter.
Aliphatic compounds
[edit]The aliphatic hydrocarbons are subdivided into three groups, homologous series according to their state of saturation: paraffins alkanes without any double or triple bonds, olefins alkenes with double bonds, which can be mono-olefins with a single double bond, di-olefins, or di-enes with two, or poly-olefins with more. The third group with a triple bond is named after the name of the shortest member of the homologue series as the acetylenes alkynes. The rest of the group is classed according to the functional groups present.
From another aspect aliphatics can be straight chain or branched chain compounds, and the degree of branching also affects characteristics, like octane number or cetane number in petroleum chemistry.
Aromatic and alicyclic compounds
[edit]Cyclic compounds can, again, be saturated or unsaturated. Because of the bonding angle of carbon, the most stable configurations contain six carbon atoms, but while rings with five carbon atoms are also frequent, others are rarer. The cyclic hydrocarbons divide into alicyclics and aromatics (also called arenes).
Of the alicyclic compounds the cycloalkanes do not contain multiple bonds, whilst the cycloalkenes and the cycloalkynes do. Typically this latter type only exists in the form of large rings, called macrocycles. The simplest member of the cycloalkane family is the three-membered cyclopropane.
Aromatic hydrocarbons contain conjugated double bonds. One of the simplest examples of these is benzene, the structure of which was formulated by Kekulé who first proposed the delocalization or resonance principle for explaining its structure. For "conventional" cyclic compounds, aromaticity is conferred by the presence of 4n + 2 delocalized pi electrons, where n is an integer. Particular instability (antiaromaticity) is conferred by the presence of 4n conjugated pi electrons.
The characteristics of the cyclic hydrocarbons are again altered if heteroatoms are present, which can exist as either substituents attached externally to the ring (exocyclic) or as a member of the ring itself (endocyclic). In the case of the latter, the ring is termed a heterocycle. Pyridine and furan are examples of aromatic heterocycles while piperidine and tetrahydrofuran are the corresponding alicyclic heterocycles. The heteroatom of heterocyclic molecules is generally oxygen, sulfur, or nitrogen, with the latter being particularly common in biochemical systems.
Rings can fuse with other rings on an edge to give polycyclic compounds. The purine nucleoside bases are notable polycyclic aromatic heterocycles. Rings can also fuse on a "corner" such that one atom (almost always carbon) has two bonds going to one ring and two to another. Such compounds are termed spiro and are important in a number of natural products.
Polymers
[edit]
One important property of carbon in organic chemistry is that it can form certain compounds, the individual molecules of which are capable of attaching themselves to one another, thereby forming a chain or a network. The process is called polymerization and the chains or networks polymers, whilst the source compound is a monomer. Two main groups of polymers exist: those artificially manufactured are referred to as industrial polymers [3] or synthetic polymers and those naturally occurring as biopolymers.
Since the invention of the first artificial polymer, bakelite, the family has quickly grown with the invention of others. Common synthetic organic polymers are polyethylene or polythene, polypropylene, nylon, teflon or PTFE, polystyrene, polyesters, polymethylmethacrylate (commonly known as perspex or plexiglas) polyvinylchloride or PVC, and polyisobutylene important artificial or synthetic rubber also the polymerised butadiene, a rubber component.
The examples are generic terms, and many varieties of each of these may exist, with their physical characteristics fine tuned for a specific use. Changing the conditions of polymerisation changes the chemical composition of the product by altering chain length, or branching, or the tacticity. With a single monomer as a start the product is a homopolymer. Further, secondary component(s) may be added to create a heteropolymer (co-polymer) and the degree of clustering of the different components can also be controlled. Physical characteristics, such as hardness, density, mechanical or tensile strength, abrasion resistance, heat resistance, transparency, colour, etc. will depend on the final composition.
Biomolecules
[edit]Biomolecular chemistry is a major category within organic chemistry. Many complex multi-functional group molecules are important in living organisms. Some are long-chain biopolymers. The main classes are carbohydrates, amino acids and proteins, polysaccharides, lipids, and nucleic acids.
Others
[edit]Organic compounds containing bonds of carbon to nitrogen, oxygen and the halogens are not normally grouped separately. Others are sometimes put into major groups within organic chemistry and discussed under titles such as organosulfur chemistry, organometallic chemistry, organophosphorus chemistry and organosilicon chemistry.
Molecular structure elucidation
[edit]
Organic compounds consist of carbon atoms, hydrogen atoms, and functional groups. The valence of carbon is 4, and hydrogen is 1, functional groups are generally 1. From the number of carbon atoms and hydrogen atoms in a molecule the degree of unsaturation can be obtained. Many, but not all structures can be envisioned by the simple valence rule that there will be one bond for each valence number. The knowledge of the chemical formula for an organic compound is not sufficient information because many isomers can exist. Organic compounds often exist as mixtures. Because many organic compounds have relatively low boiling points and/or dissolve easily in organic solvents there exist many methods for separating mixtures into pure constituents that are specific to organic chemistry such as distillation, crystallization and chromatography techniques. There exist several methods for deducing the structure an organic compound. In general usage are (in alphabetical order):
- Crystallography: This is the most precise method for determining molecular geometry; however, it is very difficult to grow crystals of sufficient size and high quality to get a clear picture, so it remains a secondary form of analysis. Crystallography has seen especially extensive use in biochemistry (for protein structure determination) and in the characterization of organometallic catalysts, which often possess significant symmetry.
- Elemental analysis: A destructive method used to determine the elemental composition of a molecule. See also mass spectrometry, below.
- Infrared spectroscopy: Chiefly used to determine the presence (or absence) of certain functional groups.
- Mass spectrometry: Used to determine the molecular weight of a compound and from the fragmentation pattern its structure. High resolution mass spectrometry can often identify the precise formula of a compound through knowledge of isotopic masses and abundances; it is thus sometimes used in lieu of elemental analysis.
- Nuclear magnetic resonance (NMR) spectrometry identifies different nuclei based on their chemical environment. This is the most important and commonly used spectroscopic technique for organic chemists, often permitting complete assignment of atom connectivity and even stereochemistry given the proper set of spectroscopy experiments (e.g. correlation spectroscopy).
- Optical rotation: Distinguishes between two enantiomers of a chiral compound based on the sign of rotation of plane polarized light. If the specific rotation of an enantiomer is known, the magnitude of rotation also gives the ratio of enantiomers in a mixed sample, though HPLC with a chiral column also can supply this information.
- UV/VIS spectroscopy: Used to determine degree of conjugation in the system. While still sometimes used to characterize molecules, UV/VIS is more commonly used to quantitate how much of a known compound is present in a (typically liquid) sample.
Additional methods are provided by analytical chemistry.
See also
[edit]- ^ Robert T. Morrison, Robert N. Boyd, and Robert K. Boyd, Organic Chemistry, 6th edition (Benjamin Cummings, 1992, ISBN 0-13-643669-2) - this is "Morrison and Boyd", a classic textbook
- ^ Richard F. and Sally J. Daley, Organic Chemistry, www.ochem4free.com, Online organic chemistry textbook.
- ^ "industrial polymers, chemistry of." Encyclopædia Britannica. 2006
