User:Kazuum/sandbox
| Renilla-luciferin 2-monooxygenase | |||||||||
|---|---|---|---|---|---|---|---|---|---|
 | |||||||||
| Identifiers | |||||||||
| EC no. | 1.13.12.5 | ||||||||
| CAS no. | 61869-41-8 | ||||||||
| Databases | |||||||||
| IntEnz | IntEnz view | ||||||||
| BRENDA | BRENDA entry | ||||||||
| ExPASy | NiceZyme view | ||||||||
| KEGG | KEGG entry | ||||||||
| MetaCyc | metabolic pathway | ||||||||
| PRIAM | profile | ||||||||
| PDB structures | RCSB PDB PDBe PDBsum | ||||||||
| Gene Ontology | AmiGO / QuickGO | ||||||||
| |||||||||
Introduction
[edit]Renilla-luciferin 2-monooxygenase, or RLuc, is a bioluminescent enzyme found in Renilla reniformis, belonging to a group of coelenterazine luciferases. Of this group of enzymes, the luciferase from Renilla reniformis has been the most extensively studied, and due to its bioluminescence requiring only molecular oxygen, has a wide range of applications, with uses as a reporter gene probe in cell culture, in vivo imaging, and various other areas of biological research[2]. Recently, chimeras of RLuc have been developed and demonstrated to be the brightest luminescent proteins to date, and have proved effective in both noninvasive single-cell and whole body imaging[3].
RLuc catalyzes the chemical reaction
Coelenterazine + O2 coelenteramide + CO2 + hν
In the process, RLuc is oxidized, and a photon of blue light is emitted.[4]
RLuc was isolated from the sea pansy Renilla reniformis, a bioluminescent sea pen. It belongs to the family of oxidoreductases, specifically those acting on single donors with O2 as the oxidant.
The systematic name of this enzyme class is Renilla-luciferin:oxygen 2-oxidoreductase (decarboxylating). Other names in common use include Renilla-type luciferase, aequorin, and luciferase (Renilla luciferin).
Biological Function
[edit]In Renilla reniformis, RLuc is found in membrane-bound intracellular structures within specialized light emitting cells[5][6], and is coupled with a closely interacting green fluorescent protein (RrGFP)[7], and a Ca++ activated luciferin binding protein (RrLBP)[8]. Although the luciferase catalyzed oxidation of coelenterazine releases a photon of blue light (480 nm), this is not observed in vivo. Instead, the energy released by the reaction involving RLuc is passed via resonance energy transfer to the fluorophore of RrGFP and emitted as a green photon (505 nm)[9], resulting in green bioluminescence observed from the animal. This process relies on a Förster resonance energy transfer (FRET) mechanism, increasing the emitted photon number approximately six-fold[10].
Structure
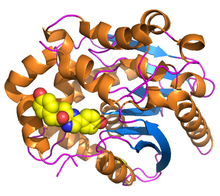
[edit]Renilla luciferase contains 311 amino acids[1], and is active as a nearly spherical single polypeptide chain monomer of 33000 - 38000 Daltons, which have a tendency for self-association, forming inactive dimers and trimers[11][12]. It has a characteristic α/β-hydrolase fold sequence at its core[13] and shares the conserved catalytic triad of residues employed by dehalogenases[14]. In RLuc, the loop containing residues 153 -163 is structurally flexible, facilitating greater diffusion of solvents into the active site, which is a catalytic triad consisting of Aspartic Acid at residue 120, Glutamic Acid at residue 144, and Histidine at residue 285[1].
Enzyme Pathway
[edit]In the membrane-bound intracellular structure containing RLuc, the apoenzyme RrLBP, and the green fluorescent protein RrGFP, a Ca2+ ion first interacts with RrLBP, causing it to release its cofactor coelenterazine[8]. Coelenterazine is then catalyzed by RLuc into coelenteramide, releasing a single photon of blue light (480 nm) in the process. This photon is captured by the adjacent (RrGFP), releasing a photon of green light. This pathway is summarized below.
Mechanism
[edit]The RLuc mediated chemical reaction involves the catalytic degradation of coelenterazine, and proceeds through a 1,2-dioxetane (also called dioxetanone or cyclic peroxide) intermediate[15]. Based on studies using radioactively labelled oxygen species within the RLuc complex, it has been determined that the luciferin carbonyl oxygen is exchanged rapidly with oxygen from water prior to incorporation of an oxygen atom from O2 via a dioxetane intermediate. The resultant CO2 also rapidly exchanges its oxygens with those from the surrounding water[16]. The general mechanism is depicted below.

References
[edit]- ^ a b c Loening, A. M.; Fenn, T. D.; Gambhir, S. S. (2007). "Crystal Structures of the Luciferase and Green Fluorescent Protein from Renilla reniformis". Journal of Molecular Biology. 374 (4): 1017–1028. doi:10.1016/j.jmb.2007.09.078. PMC 2700051. PMID 17980388.
- ^ Daunert, Sylvia (2006). Photoproteins in bioanalysis. John Wiley & Sons.
- ^ Saito, Kenta; Chang, Y-F; Horikawa, Kazuki; Hatsugai, Noriyuki; Higuchi, Yuriko; Hashida, Mitsuru; Yoshida, Yu; Matsuda, Tomoki; Arai, Yoshiyuki; Nagai, Takeharu (11 December 2012). "Luminescent proteins for high-speed single-cell and whole-body imaging". Nature Communications. 3: 1262. doi:10.1038/ncomms2248.
- ^ Schomburg, Dietmar (1994). Enzyme Handbook. Springer.
- ^ Anderson, JM; Cormier, MJ (25 April 1973). "Lumisomes, the cellular site of bioluminescence in coelenterates". The Journal of biological chemistry. 248 (8): 2937–43. PMID 4144548.
- ^ Spurlock, BO; Cormier, MJ (January 1975). "A fine structure study of the anthocodium in Renilla mülleri. Evidence for the existence of a bioluminescent organelle, the luminelle". The Journal of cell biology. 64 (1): 15–28. PMID 233975.
- ^ Ward, WW; Cormier, MJ (10 February 1979). "An energy transfer protein in coelenterate bioluminescence. Characterization of the Renilla green-fluorescent protein". The Journal of biological chemistry. 254 (3): 781–8. PMID 33175.
- ^ a b Inouye, S (March 2007). "Expression, purification and characterization of calcium-triggered luciferin-binding protein of Renilla reniformis". Protein expression and purification. 52 (1): 66–73. PMID 16997571.
- ^ Ward, William W.; Cormier, Milton J. (April 1978). "ENERGY TRANSFER VIA PROTEIN-PROTEIN INTERACTION IN RENILLA BIOLUMINESCENCE". Photochemistry and Photobiology. 27 (4): 389–396. doi:10.1111/j.1751-1097.1978.tb07621.x.
- ^ Ward, William W.; Cormier, Milton J. (September 1976). "In vitro energy transfer in Renilla bioluminescence". The Journal of Physical Chemistry. 80 (20): 2289–2291. doi:10.1021/j100561a030.
- ^ Matthews, JC; Hori, K; Cormier, MJ (11 January 1977). "Purification and properties of Renilla reniformis luciferase". Biochemistry. 16 (1): 85–91. PMID 12797.
- ^ Cormier, Milton J.; Karkhanis, Yashwant D. (January 1971). "Isolation and properties of Renilla reniformis luciferase, a low molecular weight energy conversion enzyme". Biochemistry. 10 (2): 317–326. doi:10.1021/bi00778a019.
- ^ Ollis, DL; Cheah, E; Cygler, M; Dijkstra, B; Frolow, F; Franken, SM; Harel, M; Remington, SJ; Silman, I; Schrag, J (April 1992). "The alpha/beta hydrolase fold". Protein engineering. 5 (3): 197–211. PMID 1409539.
- ^ Loening, AM; Fenn, TD; Wu, AM; Gambhir, SS (September 2006). "Consensus guided mutagenesis of Renilla luciferase yields enhanced stability and light output". Protein engineering, design & selection : PEDS. 19 (9): 391–400. PMID 16857694.
- ^ Anderson, JM; Charbonneau, H; Cormier, MJ (12 March 1974). "Mechanism of calcium induction of Renilla bioluminescence. Involvement of a calcium-triggered luciferin binding protein". Biochemistry. 13 (6): 1195–200. PMID 4149963.
- ^ Hart, Russell C.; Stempel, Kerstin E.; Boyer, Paul D.; Cormier, Milton J. (April 1978). "Mechanism of the enzyme-catalyzed bioluminescent oxidation of coelenterate-type luciferin". Biochemical and Biophysical Research Communications. 81 (3): 980–986. doi:doi:10.1016/0006-291X(78)91447-X.
{{cite journal}}: Check|doi=value (help)
Category:EC 1.13.12
Category:Enzymes of known structure



![{\displaystyle RrLBP{\ce {->[{\ce {+Ca^{2+}}}]}}apoRrLBP(+Ca^{2+})+coelenterazine}](https://wikimedia.org/api/rest_v1/media/math/render/svg/9e740022b3d02af4cce0c187b65558ad9d30acd3)
![{\displaystyle coelenterazine+O2{\ce {->[{\ce {RLuc}}]}}coelenteramide+CO2+hv(480nm)}](https://wikimedia.org/api/rest_v1/media/math/render/svg/b074c627fa2a1299751d41bfa8658f8092fb81ac)
![{\displaystyle hv(480nm){\ce {->[{\ce {RrGFP}}]}}hv(505nm)}](https://wikimedia.org/api/rest_v1/media/math/render/svg/cdcd4f740cf085b4cc2a4f732ffe1c8f870f078d)