User:Jaskaran33/Recrystallization (chemistry)
| This is the sandbox page where you will draft your initial Wikipedia contribution.
If you're starting a new article, you can develop it here until it's ready to go live. If you're working on improvements to an existing article, copy only one section at a time of the article to this sandbox to work on, and be sure to use an edit summary linking to the article you copied from. Do not copy over the entire article. You can find additional instructions here. Remember to save your work regularly using the "Publish page" button. (It just means 'save'; it will still be in the sandbox.) You can add bold formatting to your additions to differentiate them from existing content. |
Article Draft
[edit]Recrystallization purifies chemicals by dissolving a mixture of a compound and its impurities in an appropriate solvent prior to temperature adjustment.Following the adjustment, the mixture will separate yielding a crystallized compound and impurities. The name originates from crystals often forming upon precipitation. Recrystallization can as well refer to the natural growth of larger ice crystals at the expense of smaller ones.
Chemistry
[edit]In chemistry, recrystallization: 127–132 is a technique utilized for compound purification. The various methods of recrystallization, including how to induce such when crystals appear not to form upon cooling,are outlined below:
Single-solvent recrystallization
[edit]A mixture of "compound A" and "impurity B" typically dissolves in a minute amount of hot solvent. The solution will then passively cool lowering the solubility of the compounds in solution resulting in the recrystallization of "compound A" absent of "impurity B".


In an ideal situation the solubility product of impurity B is not exceeded at any temperature during recrystallization. In that case, the solid crystals will consist of pure A and all the impurities will remain in the solution. The solid crystals are then collected by filtration and the filtrate is discarded. If the solubility product of the impurity is exceeded, some of the impurities will co-precipitate. However, because of the relatively low concentration of the impurity, its concentration in the precipitated crystals will be less than its concentration in the original solid. Repeated recrystallization will result in an even purer crystalline precipitate. The purity is then checked after each recrystallization by melting point measurements, since impurities are expected to lower the melting point of a pure compound. NMR spectroscopy can as well be conducted for purity assessment. Repeated recrystallization results in some loss of material because of the non-zero solubility of compound A.
Multi-solvent recrystallization
[edit]This method is the same as the above but here two solvents are to be used. This method relies on the impure product being soluble in one solvent when it is heated while being fully insoluble in the other regardless of temperature. The proportion of the first and second solvent is critical. Here the terms first and second are in reference to soluble and insoluble solvents respectively. Typically the second solvent, following the dissolvation of the impure solid in the heated first solvent, is added slowly until the desired product begins to crystallize from the solution. Following this, the solution will be cooled further inducing crystallization.
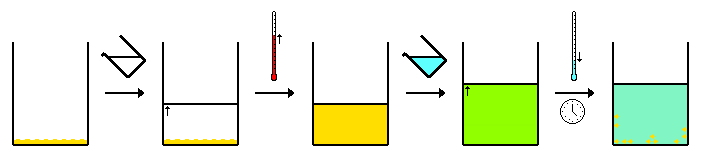
(Beginning of verification) The reverse of this method can be used where a mixture of solvents dissolves both A and B. One of the solvents is then removed by distillation or by an applied vacuum. This results in a change in the proportions of the solvent causing either "compound A" or "impurity B" to precipitate.(End of verification)
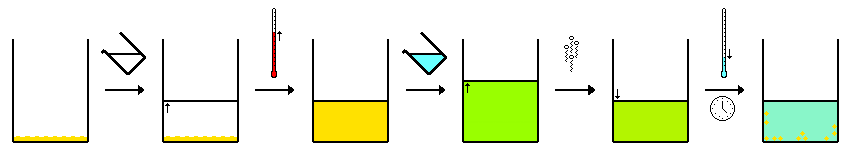
Hot filtration-recrystallization
[edit]Hot filtration recrystalization can be used to separate "compound A" from both "impurity B" and some "insoluble matter C". This technique normally uses a single-solvent system as described above. When both "compound A" and "impurity B" are dissolved in the minimum amount of hot solvent, the solution is filtered to remove "insoluble matter C". This matter may be anything from a third impurity compound to fragments of broken glass. For a successful procedure, one must ensure that the filtration apparatus is hot in order to stop the dissolved compounds from crystallizing from the solution during filtration, thus forming crystals on the filter paper or funnel.
One way to achieve this is to heat a conical flask containing a small amount of clean solvent on a hot plate. A filter funnel is rested on the mouth, and hot solvent vapors keep the stem warm. Jacketed filter funnels may also be used. The filter paper is preferably fluted, rather than folded into a quarter; this allows quicker filtration, thus less opportunity for the desired compound to cool and crystallize from the solution.
Often it is simpler to do the filtration and recrystallization as two independent and separate steps. That is dissolve "compound A" and "impurity B" in a suitable solvent at room temperature, filter (to remove insoluble compound/glass), remove the solvent and then recrystallize using any of the methods listed above.

Seeding
[edit]Crystallization requires an initiation step. This can be spontaneous or can be done by adding a small amount of the pure compound (a seed crystal) to the saturated solution, or can be done by simply scratching the glass surface to create a seeding surface for crystal growth. It is thought that even dust particles can act as simple seeds.
Single perfect crystals (for X-ray analysis)
[edit]Growing crystals for X-ray crystallography can be difficult. For X-ray analysis, single perfect crystals are required. Typically a small amount (5–100 mg) of a pure compound is used, and crystals are allowed to grow very slowly. Several techniques can be used to grow these perfect crystals:
- Slow evaporation of a single solvent - typically the compound is dissolved in a suitable solvent and the solvent is allowed to slowly evaporate. Once the solution is saturated crystals can be formed.
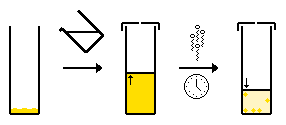
- Slow evaporation of a multi-solvent system - the same as above, however as the solvent composition changes due to evaporation of the more volatile solvent. The compound is more soluble in the volatile solvent, and so the compound becomes increasingly insoluble in solution and crystallizes.

- Slow diffusion - similar to the above. However, a second solvent is allowed to evaporate from one container into a container holding the compound solution (gas diffusion). As the solvent composition changes due to an increase in the solvent that has gas diffused into the solution, the compound becomes increasingly insoluble in the solution and crystallizes.
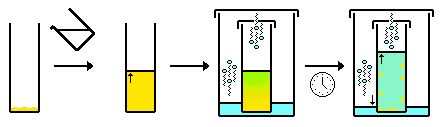
- Interface/slow mixing (often performed in an NMR tube). Similar to the above, but instead of one solvent gas-diffusing into another, the two solvents mix (diffuse) by liquid-liquid diffusion. Typically a second solvent is "layered" carefully on top of the solution containing the compound. Over time the two solution mix. As the solvent composition changes due to diffusion, the compound becomes increasingly insoluble in solution and crystallizes, usually at the interface. Additionally, it is better to use a denser solvent as the lower layer, and/or a hotter solvent as the upper layer because this results in the slower mixing of the solvents.

- Specialized equipment can be used in the shape of an "H" to perform the above, where one of the vertical lines of the "H" is a tube containing a solution of the compound, and the other vertical line of the "H" is a tube containing a solvent which the compound is not soluble in, and the horizontal line of the "H" is a tube which joins the two vertical tubes, which also has a fine glass sinter that restricts the mixing of the two solvents.

- Once single perfect crystals have been obtained, it is recommended that the crystals are kept in a sealed vessel with some of the liquid of crystallization to prevent the crystal from 'drying out'. Single perfect crystals may contain solvent of crystallization in the crystal lattice. Loss of this internal solvent from the crystals can result in the crystal lattice breaking down, and the crystals turning to powder.
Ice
[edit]For ice, recrystallization refers to the growth of larger crystals at the expense of smaller ones. Some biological antifreeze proteins have been shown to inhibit this process, and the effect may be relevant in freezing-tolerant organisms.[1]
See also
[edit]- Craig tube, an apparatus for small-scale recrystallization
- Crystal structure
- Crystallization
- Fractional crystallization (chemistry)
- Laser-heated pedestal growth
- Seed crystal
- Single crystal
References
[edit]- ^ Kumar Verma, Ashok (2014). "Recrystallization of Ice". Encyclopedia of Snow, Ice and Glaciers. Encyclopedia of Earth Sciences Series. p. 932. doi:10.1007/978-90-481-2642-2_439. ISBN 978-90-481-2641-5.
Cite error: A list-defined reference with the name "Harwood_Moody" has been invoked, but is not defined in the <references> tag (see the help page).
Reference books
[edit]- Laurence M. Harwood; Christopher J. Moody; Jonathan M. Percy (1999). Experimental organic chemistry: standard and microscaling. Wiley. ISBN 9780632048199.
- John Leonard; B. Lygo; Garry Procter (2 June 1994). Advanced practical organic chemistry. CRC Press. ISBN 9780748740710.
