User:JSdeutsch/Trichoplax adhaerens
[This is the 1 October 2008 English rendering of the German Wikipedia article Trichoplax adhaerens as used for the English Wikipedia article Trichoplax (which has undergone considerable modification since then). Translator's notes can be found under the discussion tab above.]
| Trichoplax adhaerens | |
|---|---|
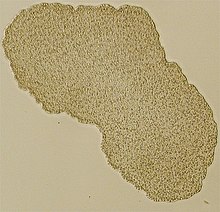
| |
| Light microscope image of Trichoplax (specimen ca. 0.5 mm across) | |
| Scientific classification | |
| Kingdom: | |
| Subkingdom: | |
| Superphylum: | |
| Phylum: | Placozoa
|
| Genus: | Trichoplax
|
| Species: | adhaerens
|
| Binomial name | |
| Trichoplax adhaerens F.E. von Schultze, 1883
| |
Trichoplax adhaerens is the only known species of Placozoa and was discovered in 1883 by the German zoologist Franz Eilhard Schulze in a seawater aquarium at the Zoological Institute in Graz, Austria. The genus name he gave the animal is derived from the classical Greek θρίξ (thrix), "hair", and πλάξ (plax), "plate"; the Latin species name adhaerens means "adhering"; so the binomial name can be translated roughly as "adhering hairy plate".
The Placozoa are the simplest in structure of all multicellular animals (Metazoa) and with only one species, Trichoplax adhaerens, form a phylum of their own. A common name does not yet exist for the taxon; the scientific name literally means "flat animals".[1]
For a long time these organisms, which had not been observed in their natural habitat, were considered to be juvenile stages of nettle-like animals (Cnidaria): for more than a half century they were interpreted as planula larvae of the hydrozoan species Eleutheria krohni. It was research in the 1970s by the Tübingen protozoologist Karl Gottlieb Grell and others that first elucidated the early phases of the animals' embryonic development and contributed considerably to establishing a separate phylum for them. The species is formally assigned to the family Trichoplacidae.
Structure
[edit]As their name implies, Placozoa generally have a thinly flattened, plate-like body. Its diameter is often less than half a millimeter but occasionally measures more than two or three millimeters; the body is usually only about 25 microns thick. These gray organisms, transparent in back-lighting, are in most cases just visible to the naked eye. Superficially they look like large amoebas and, like the latter, continually change their external shape. In addition, spherical phases occasionally appear that may facilitate passive transport to new habitats.
As its assignment to the Parazoa suggests, Trichoplax adhaerens lacks tissues and organs; there is also no manifest body symmetry, so it is not possible to distinguish front from back or left from right.
Epitheloid
[edit]Both structurally and functionally, it is possible to distinguish a back or dorsal side from a belly or ventral side in Trichoplax adhaerens. Both consist of a single layer of cells coated on the outside with slime and are reminiscent of epithelial tissue, primarily due to the junctions — belt desmosomes — between the cells. In contrast to true epithelium, however, the cell layers of the Placozoa possess no basal lamina, which refers to a thin layer of extracellular material underlying epithelium that stiffens it and separates it from the body's interior. The absence of this structure, which is otherwise to be found in all animals except the sponges, can be explained in terms of function: a rigid separating layer would make the amoeboid changes in the shape of Trichoplax adhaerens impossible. Instead of an epithelium, therefore, we speak of an epitheloid in the Placozoa.
A mature individual consists of up to a thousand cells that can be divided into four different cell types. The monociliated cells of the dorsal epitheloid are flattened and contain lipid bodies. The cells on the ventral side are likewise just monociliated but have an elongated columnar form of small cross section at the surface, causing the cilia to be very closely spaced on the ventral side and to form a ciliated "crawling sole". Between them are found unciliated gland cells thought to be capable of synthesizing digestive enzymes.
Fiber syncytium
[edit]Between the two layers of cells is a liquid-filled interior space, which, except for the immediate zones of contact with the central and dorsal sides, is pervaded by a star-shaped fiber syncytium: a fibrous network that consists essentially of a single cell but contains numerous nuclei that, while separated by internal crosswalls (septa), do not have true cell membranes between them. Similar structures are also found in the sponges (Porifera) and many fungi.
On both sides of the septa are liquid-filled capsules that cause the mentioned separating structures to resemble synapses, i.e. nerve-cell junctions that occur in fully expressed form only in animals with tissues (Eumetazoa). Striking accumulations of calcium ions, which may have a function related to the propagation of stimuli, likewise suggest a possible role as protosynapses. This view is supported by the fact that fluorescent antibodies against cnidarian neurotransmitters, i.e. precisely those signal carriers that are transferred in synapses, bind in high concentrations in certain cells of Trichoplax adhaerens and thus indicate the existence of comparable substances in the Placozoa. In addition, the fiber syncytium contains molecules of actin and probably also of myosin, which occur in the muscle cells of eumetazoans. In the placozoans, they ensure that the individual fibers can relax or contract and thus help determine the animals' shape.
In this way, the fiber syncytium assumes the functions of nerve and muscle tissues. Moreover, at least a portion of digestion occurs here. On the other hand, no gelatinous extracellular matrix exists of the kind observed, as mesoglea, in cnidarians and ctenophores.
Pluripotent cells, which can differentiate into other cell types, have not yet been demonstrated unambiguously as such, in contrast to the case of the Eumetazoa. The conventional view is that dorsal and ventral epithelium cells arise only from their own kind.
Genetics
[edit]All nuclei of placozoan cells contain twelve chromosomes that are only about two to three microns in size. Three pairs are metacentric, and the others are acrocentric, meaning that the centromere, the attachment point for the spindle fibers in cell division, is located at either the center or the extreme end of each chromosome. The cells of the fiber syncytium can be tetraploid, i.e. contain a quadruple complement of chromosomes.
A single complement of chromosomes in Trichoplax adhaerens contains a total of less that fifty million base pairs and thus forms the smallest animal genome; the number of base pairs in the intestinal bacterium Escherichia coli is smaller by a factor of only ten.
The genetic complement of Trichoplax adhaerens has not yet been very well researched; it has, however, already been possible to identify several genes, such as Brachyury and Tbx2/3, which are homologous to corresponding base-pair sequences in eumetazoans. Of particular significance is Trox-2, a placozoan gene known under the name Cnox-2 in cnidarians and as Gsx in the bilaterally symmetrical Bilateria. As a homeobox or Hox gene it plays a role in organization and differentiation along the axis of symmetry in the embryonic development of eumetazoans; in cnidarians, it appears to determine the position of mouth-facing (oral) and opposite-facing (aboral) sides of the organism. As noted, however, placozoans possess no axes of symmetry. Thus exactly where the gene is transcribed in the body of Trichoplax is of special interest. Antibody studies have been able to show that the gene's product occurs only in the transition zones of the dorsal and ventral sides, perhaps in a fifth cell type that has not yet been characterized. It is not yet clear whether these cells, contrary to traditional views, are stem cells, which play a role in cell differentiation. In any case, Trox-2 can be considered a possible candidate for a proto-Hox gene, from which the other genes in this important family could have arisen through gene duplication and variation.
Initially, molecular-biology methods were applied unsuccessfully to test the various theories regarding Placozoa's position in the Metazoa system. No clarification was achieved with standard markers such as 18S rDNA/RNA: the marker sequence was apparently "garbled", i.e. rendered uninformative as the result of many mutations. Nevertheless, this negative result supported the suspicion that Trichoplax might represent an extremely primitive lineage of metazoans, since a very long period of time had to be assumed for the accumulation of so many mutations.
The truly interesting question as to whether the Placozoa could be older than the sponges appears to have been answered recently by elucidation of the mitochondrial genome. The mitochondrial genome shows Trichoplax to be distinctly more primitive than the sponges, since overall it is more reminiscent of conditions found in unicellular species (Dellaporta et al. 2006). This implies that the Placozoa would have arisen relatively soon after the evolutionary transition from unicellular to multicellular forms. Specifically, this result supports a hypothesis of placozoan ancestry based on a functional-morphology interpretation of its structure and at the same time refutes the earlier, purely anatomy-based interpretation according to which sponges were considered to be older (for concurring morphological theories, see the section on "Phylogenesis").
Distribution and habitat
[edit]Precise information about distribution does not exist for the Placozoa, but these animals have been observed, among other places, in the Red Sea, the Mediterranean, and the Caribbean, off Hawaii, Guam, Samoa, Japan, Vietnam and Papua New Guinea, and on the Great Barrier Reef off the east coast of Australia. Trichoplax adhaerens is "found" regularly in seawater aquaria, such as in Plymouth in southern England and in Miami, Florida.
Field specimens have come from the coastal tidal zones of tropical and subtropical seas, where these animals are found on such substrates as the trunks and roots of mangroves, shells of mollusks, fragments of stony corals or simply on pieces of rock. One study was able to detect seasonal population fluctuations, although their causes have not yet been found.
Feeding and symbionts
[edit]
Trichoplax adhaerens feeds on small algae, particularly on green algae (Chlorophyta) of the genus Chlorella, cryptomonads (Cryptophyta) of the genera Cryptomonas and Rhodomonas, and blue-green bacteria (Cyanobacteria) such as Phormidium inundatum, but also on detritus from other organisms. One or several small pockets form around particles of nutrients for this purpose on the ventral side, into which digestive enzymes are released by the gland cells; the organisms thus develop a temporary "external stomach", so to speak. The enclosed nutrients are then taken up by pinocytosis ("cell-drinking") by the ciliated cells located on the ventral surface.
Nutrient particles, e.g. entire single-celled organisms, can also be ingested through the upper epitheloid (that is, the "dorsal surface" of the animal). This mode of feeding could be unique in the animal kingdom: The particles, collected in a slime layer, are drawn through the intercellular gaps (cellular interstices) of the epitheloid by the fiber cells and then digested by phagocytosis ("cell-eating"). Such "collecting" of nutrient particles through an intact tegument is only possible because some "insulating" elements (specifically, a basal lamina under the epitheloid and certain types of cell-cell junctions) are not present in the Placozoa.
Not all bacteria in the interior of Placozoa are digested as food: in the endoplasmic reticulum, an organelle of the fiber syncytium, bacteria are frequently found that appear to live in symbiosis with Trichoplax adhaerens.
Locomotion
[edit]Placozoa can move in two different ways on solid surfaces: First, their ciliated crawling sole lets them glide slowly across the substrate; second, they can change location by modifying their body shape, as an amoeba does. These movements are not centrally coordinated, since no muscle or nerve tissues exist. It can happen that an individual moves simultaneously in two different directions and consequently divides into two parts.
It has been possible to demonstrate a close connection between body shape and the speed of locomotion, which is also a function of available food:
- At low nutrient density, the spread-out area fluctuates slightly but irregularly; speed remains relatively constant at about 15 microns per second.
- If nutrient density is high, however, the area covered oscillates with a stable period of about 8 minutes, in which the greatest extent reached by the organism can be as much as twice the smallest. Its speed, which remains consistently below 5 microns per second, varies with the same period. In this case, a high speed always corresponds to a reduced area, and vice versa.
Since the transition is not smooth but happens abruptly, the two modes of extension can be very clearly separated from one another. As a simplification, Trichoplax adhaerens can be modeled as a nonlinear dynamic system that is far from thermodynamic equilibrium.
The following is a qualitative explanation of the animal's behavior:
- At low nutrient density, Trichoplax maintains a constant speed in order to uncover food sources without wasting time.
- Once such a source is identified by high nutrient density, the organism increases its area in regular increments and thereby enlarges the surface in contact with substrate. This enlarges the surface through which nutrients can be ingested. The animal reduces its speed at the same time in order to actually consume all of the available food.
- Once this is nearly completed, Trichoplax reduces its area again to move on. Because food sources such as algal mats are often relatively extensive, it is reasonable for such an animal to stop moving after a brief period in order to flatten out again and absorb nutrients. Thus Trichoplax progresses relatively slowly in this phase.
The actual direction in which Trichoplax moves each time is random: if we measure how fast an individual animal moves away from an arbitrary starting point, we find a linear relationship between elapsed time and mean square distance between starting point and present location. Such a relationship is also characteristic of random Brownian motion, which thus can serve as a model for locomotion in the Placozoa.
Small animals are also capable of swimming actively with the aid of their cilia. As soon as they come into contact with a possible substrate, a dorsoventral response occurs: the dorsal cilia continue to beat, whereas the cilia of ventral cells stop their rhythmic beating. At the same time, the ventral surface tries to make contact with the substrate; small protrusions and invaginations, the microvilli found on the surface of the columnar cells, help in attaching to the substrate via their adhesive action.
Regeneration
[edit]A notable characteristic of the Placozoa is that they can regenerate themselves from extremely small groups of cells. Even when large portions of the organism are removed in the laboratory, a complete animal develops again from the remainder. It is also possible to rub Trichoplax adhaerens through a strainer in such a manner that individual cells are not destroyed but are separated from one another to a large extent. In the test tube they then find their way back together again to form complete organisms. If this procedure is performed on several previously stained individuals simultaneously, the same thing occurs. In this case, however, cells that previously belonged to a particular individual can suddenly show up as part of another.
Propagation
[edit]The Placozoa normally propagate asexually. The animal pinches itself off in the middle for this purpose, producing two roughly equal-sized daughters, although these remain loosely connected for a while after fission. More rarely, budding processes are observed: spherules of cells separate from the dorsal surface; each of these combines all known cell types and subsequently grows into an individual of its own.
Sexual reproduction is thought to be triggered by excessive population density. As a result, the animals absorb liquid, begin to swell, and separate from the substrate so that they float freely in the water. In the protected interior space, the ventral cells form an ovum surrounded by a special envelope, the fertilization membrane; the ovum is supplied with nutrients by the surrounding syncytium, allowing energy-rich yolk to accumulate in its interior. Once maturation of the ovum is complete, the rest of the animal degenerates, liberating the ovum itself. Small unciliated cells that form at the same time are interpreted to be spermatozoa. It has not yet been possible to observe fertilization itself; the existence of the fertilization membrane is currently taken to be evidence, however, that it has taken place.
Usually even before its liberation, the ovum initiates cleavage processes in which it becomes completely pinched through at the middle. A ball of cells characteristic of animals, the blastula, is ultimately produced in this manner, with a maximum of 64 cells. Development beyond this 64-cell stage has not yet been observed.
Due to the possibility of its cloning itself by asexual propagation without limit, the life span of Placozoa is potentially infinite; in the laboratory, several lines descended from a single organism have been maintained in culture for an average of 20 years without the occurrence of sexual processes.
Role as a model organism
[edit]Long ignored as an exotic, marginal phenomenon, Trichoplax adhaerens is today viewed as a potential biological model organism. In particular, research is needed to determine how a group of cells that cannot be considered full-fledged epithelial tissue organizes itself, how locomotion and coordination occur in the absence of true muscle and nerve tissue, and how the absence of a concrete body axis affects the animal's biology. At the genetic level, the way in which Trichoplax adhaerens protects against damage to its genome needs to be studied, particularly with regard to the existence of special DNA-repair processes. Complete decoding of the genome should also clarify the placozoans' place in evolution, which continues to be controversial.
In addition to basic research, this animal could also be suitable for studying wound-healing and regeneration processes; as yet unidentified metabolic products should be researched for potential pharmaceutical applications. Finally, Trichoplax adhaerens is also being considered as an animal model for testing pharmaceutical compounds.
Phylogenesis
[edit]The phylogenetic affinities of the Placozoa are subject to debate. Fossil records do not exist and, due to the soft body, are not to be expected either, so the position of Trichoplax adhaerens will have to be revealed solely through the comparison of modern species.
Their traditional assignment to Parazoa is not based on accepted genetic affinities of the Placozoa but instead classifies these animals according to their level of organization: like the sponges (Porifera) with which they are combined in this taxon, they possess no tissues or organs; the epitheloid is not viewed as a true tissue in this respect. Yet these are "primitive features", referred to as symplesiomorphies, inherited from the latest common ancestor of all animals and thus, according the phylogenetic systematics currently advocated by many biologists, cannot be used as the basis for evolutionary affinity.
Functional-morphology hypothesis
[edit]
On the basis of their simple structure, the Placozoa are frequently viewed as a model organism for the transition from unicellular organisms to the multicellular animals (Metazoa) and are thus considered a sister taxon to all other metazoans:
Multicellular animals (Metazoa) ├─Placozoa └─unnamed ├─Sponges (Porifera) └─Animals with tissues (Eumetazoa)
According to a functional-morphology model, all or most animals are descended from a gallertoid, a free-living (pelagic) sphere in seawater, consisting of a single ciliated layer of cells supported by a thin, noncellular separating layer, the basal lamina. The interior of the sphere is filled with contractile fibrous cells and a gelatinous extracellular matrix. Both the modern Placozoa and all other animals then descended from this multicellular beginning stage via two different processes:
- Infolding of the epithelium led to the formation of an internal system of ducts and thus to the development of a modified gallertoid from which the sponges (Porifera), Cnidaria and Ctenophora subsequently developed.
- Other gallertoids, according to this model, made the transition over time to a benthic mode of life; that is, their habitat has shifted from the open ocean to the floor (benthic zone). While the probability of encountering food, potential sexual partners, or predators is the same in all directions for animals floating freely in the water, there is a clear difference on the seafloor between the sides facing toward and away from the substrate, and between their orientation and the vertical direction perpendicular to the substrate. This results naturally in a selective advantage for flattening of the body, as of course can be seen in many benthic species. In the proposed functional-morphology model, the Placozoa, and possibly also several organisms known only from the fossil state, are descended from such a life form, which is now termed placuloid. Three different life strategies have accordingly led to three different lines of development:
- Animals that live interstitially in the sand of the ocean floor were responsible for the fossil crawling traces that are considered the earliest evidence of animals and are detectable even prior to the dawn of the Ediacaran Period in geology. These are usually attributed to bilaterally symmetrical worms, but the hypothesis presented here views animals derived from placuloids, and thus close relatives of Trichoplax adhaerens, to be the producers of the traces.
- Animals that incorporated algae as photosynthetically active endosymbionts, i.e. primarily obtaining their nutrients from their partners in symbiosis, were accordingly responsible for the mysterious creatures of the Ediacara fauna that are not assigned to any modern animal taxon and lived during the Ediacaran Period, before the start of the Paleozoic.
- Animals that grazed on algal mats were ultimately the direct ancestors of the Placozoa. The advantages of an amoeboid multiplicity of shapes thus allowed a previously present basal lamina and a gelatinous extracellular matrix to be lost secondarily. Pronounced differentiation between the ventral surface facing the substrate and the dorsal, facing away from it, accordingly led to the physiologically distinct cell layers of Trichoplax adhaerens that can still be seen today. Consequently, these are analogous, but not homologous, to ectoderm and endoderm, the "external" and "internal" cell layers in eumetazoans; i.e. the structures corresponding functionally to one another have, according to the proposed hypothesis, no common evolutionary origin.
Should the analysis presented above turn out to be correct, Trichoplax adhaerens would be the oldest branch of the multicelled animals and a relic of the Ediacara fauna, or even the pre-Ediacara fauna. Due to the absence of extracellular matrix and basal lamina, the development potential of these animals, very successful in their ecological niche, was of course limited, which would explain the low rate of evolution, referred to as bradytely, of their phenotype, their outward form as adults.
Epitheliozoa hypothesis
[edit]Functional-morphology hypotheses are not undisputed among scientists and are often rejected because of their highly theoretical character, which is not directly accessible to empirical study. Cladistics, a modern form of systematics research, is based exclusively on demonstrable features of living and fossil animal groups (taxa) for reconstructing the genealogy of a species or group.
The most important concept based on purely morphological characteristics pictures the Placozoa as the nearest relative of the animals with true tissues (Eumetazoa). The taxon they share, called the Epitheliozoa, is itself construed to be a sister group to the sponges (Porifera):
Metazoa ├─Porifera └─Epitheliozoa ├─Placozoa └─Eumetazoa
The principle support for such a relationship comes from special cell/cell junctions, the belt desmosomes, that occur not just in the Placozoa but in all animals except the sponges; they enable the cells to join together in an unbroken layer like the epitheloid of the Placozoa. Trichoplax adhaerens also shares the ventral gland cells with most eumetazoans. Both characteristics can be considered apomorphies, i.e. evolutionarily derived features, and thus form the basis of a common taxon for all animals that possess them.
One possible scenario inspired by the proposed hypothesis starts with the idea that the monociliated cells of the epitheloid in Trichoplax adhaerens evolved by reduction of the collars in the collar cells (choanocytes) of sponges as the ancestors of the Placozoa abandoned a filtering mode of life. The epitheloid would then have served as the precursor to the true epithelial tissue of the eumetazoans.
In contrast to the model based on functional morphology described earlier, in the Epitheliozoa concept the ventral and dorsal cell layers of the Placozoa are homologs of endoderm and ectoderm, the two basic embryonic cell layers of the eumetazoans — the digestive gastrodermis in the Cnidaria or the gut epithelium in the bilaterally symmetrical Bilateria may have developed from endoderm, whereas ectoderm is, among other things, the precursor to the external skin layer (epidermis). The interior space pervaded by a fiber syncytium in the Placozoa would then correspond to connective tissue in the other animals. It is uncertain whether the calcium ions stored in the syncytium are related to the lime skeletons of many cnidarians.
Eumetazoa hypothesis
[edit]A third hypothesis, based primarily on molecular genetics, views the Placozoa as highly simplified eumetazoans. According to this, Trichoplax adhaerens is descended from considerably more complex animals that already had muscles and nerve tissues. Both tissue types, as well as the basal lamina of the epithelium, were accordingly lost more recently by radical secondary simplification.
Various studies in this regard so far yield differing results for identifying the exact sister group: in one case the Placozoa would qualify as the nearest relatives of the Cnidaria, while in another they would be a sister group to the Ctenophora, and occasionally they are placed directly next to the Bilateria:
Metazoa
├─Porifera
└─Eumetazoa
├─Ctenophora
└─unnamed
├─Cnidaria
└─unnamed
├─Placozoa
└─Bilateria
Another proposal, to place them within Cnidaria, is currently the only one that can be ruled out with high probability.
An argument raised against the proposed scenario is that it leaves morphological features of the animals completely out of consideration. The extreme degree of simplification that would have to be postulated for the Placozoa in this model, moreover, is known only for parasitic organisms but would be difficult to explain functionally in a free-living species like Trichoplax adhaerens.
Systematics
[edit]Currently, only one species, Trichoplax adhaerens, is acknowledged to belong to the Placozoa. To be sure, the Italian Francesco Saverio Monticelli described another species in 1893, named Treptoplax reptans, which he found in the waters around Naples. It seems to have gone missing since 1896; its existence is doubted by most zoologist today.
Because great genetic differences often occur between representatives of Trichoplax adhaerens, differences that in other taxa would result in their being spread among different genera, it is currently unclear whether the single species based on morphological criteria does not actually correspond to a group of crypto-species, i.e. species that are not outwardly distinguishable from one another. Distribution of the genetic variants is not a function of geography: some variants are found in multiple regions (e.g. Pacific, Caribbean and Red Sea). At the same time, very different genetic variants can be isolated from the same habitat.
References
[edit]- ^ Rüdiger Wehner, Walter Gehring: Zoologie. 24th Edition, Thieme, Stuttgart, June 2007, p. 696
Further reading
[edit]- Edward E. Ruppert, R. S. Fox, R. D. Barnes: Invertebrate Zoology – a functional evolutionary approach. ch. 5. Brooks/Cole, London 2004 (7th ed.), p. 94, ISBN 0-03-025982-7
- Richard C. Brusca, G. J. Brusca: Invertebrates. ch. 7. Sinauer Associates, Sunderland Mass 2002 (2nd ed.), p. 210, ISBN 0878930973
Scientific literature
[edit]- V. J. Birstein: "On the Karyotype of Trichoplax sp. (Placozoa)." in: Biologisches Zentralblatt. Fischer, Jena – Stuttgart 108 (1989), p. 63, ISSN 0006-3304
- K. G. Grell, A. Ruthmann: "Placozoa." in: F. W. Harrison, J. A. Westfall (eds.): Microscopic Anatomy of Invertebrates. Vol. 2. Wiley-Liss, New York 1991, p. 13, ISBN 0471562246
- W. Jakob, S. Sagasser, S. Dellaporta, P. Holland, K. Kuhn, B. Schierwater: "The Trox-2 Hox/Para Hox gene of Trichoplax (Placozoa) marks an epithelial boundary." in: Development Genes and Evolution. Springer, Berlin 214 (2004), p. 170, ISSN 0949-944X
- Y. K. Maruyama: "Occurrence in the Field of a Long-Term, Year-Round Stable Population of Placozoa." in: Biological Bulletin. Laboratory, Woods Hole Mass 206:1 (2004), p. 55, ISSN 0006-3185
- T. Syed, B. Schierwater: "The evolution of the Placozoa – A new morphological model." in: Senckenbergiana lethaea. Schweizerbart, Stuttgart 82:1 (2002), p. 315, ISSN 0037-2110
- P. Schubert: "Trichoplax adhaerens (Phylum Placozoa) has cells that react with antibodies against the neuropeptide RFAmide." in: Acta Zoologica. Blackwell Science, Oxford 74:2, (1993), p. 115, ISSN 0001-7272
- T. Ueda, S. Koga, Y. K. Marayama: "Dynamic patterns in the locomotion and feeding behaviour by the placozoan Trichoplax adhaerens." in: BioSystems. North-Holland Publ., Amsterdam 54 (1999), p. 65, ISSN 0303-2647
- O. Voigt, A.G. Collins, V. Buchsbaum Pearse, J.S. Pearse, A. Ender, H. Hadrys, B. Schierwater: "Placozoa – no longer a phylum of one." in: Current Biology. Cell Press, Cambridge Mass 14:22 (2004), p. R944, ISSN 0960-9822
First descriptions
[edit]- Placozoa
- K. G. Grell: "Trichoplax adhaerens, F. E. Schulze und die Entstehung der Metazoen." ("Trichoplax adhaerens, F.E. Schulze, and the evolution of the metazoans") in: Naturwissenschaftliche Rundschau. Wiss. Verl.-Ges., Stuttgart 24 (1971), p. 160, ISSN 0028-1050
- Treptoplax reptans
- F. S. Monticelli: "Treptoplax reptans n. g., n. s." in: Rendiconti / Accademia Nazionale dei Lincei, Roma, Classe di Scienze Fisiche, Matematiche e Naturali. Rome 2:5 (1893), p. 39, ISSN 0001-4435
- Trichoplax adhaerens
- F. E. Schulze: "Trichoplax adhaerens n. g., n. s." in: Zoologischer Anzeiger. Elsevier, Amsterdam-Jena 6 (1883), p. 92, ISSN 0044-5231
