User:Immunize/Infectious causes for a fever
Here is a list of infectious causes of fever It is in my userspace for improvement and may be moved back in to the mainspace in the future.
List
[edit]Diptheria
[edit]Diphtheria[1] is a contagious disease spread by direct physical contact or breathing the aerosolized secretions of infected individuals. Historically quite common, diphtheria has largely been eradicated in industrialized nations through widespread vaccination. In the United States for example, there were 52 reported cases of diphtheria between 1980 and 2000; between 2000 and 2007 there were only three cases[2] as the DPT (Diphtheria–Pertussis–Tetanus) vaccine is recommended for all school-age children. Boosters of the vaccine are recommended for adults since the benefits of the vaccine decrease with age without constant re-exposure; they are particularly recommended for those traveling to areas where the disease has not been eradicated.
The respiratory form has an incubation period of 2–5 days. The onset of disease is usually gradual. Symptoms include fatigue, fever, a mild sore throat and problems swallowing. Children infected have symptoms that include nausea, vomiting, chills, and a high fever, although some do not show symptoms until the infection has progressed further. In 10% of cases, patients experience neck swelling, informally referred to as "bull neck." These cases are associated with a higher risk of death.
Tetanus
[edit]Tetanus,[3] also called lockjaw, is a medical condition characterized by a prolonged contraction of skeletal muscle fibers. The primary symptoms are caused by tetanospasmin, a neurotoxin produced by the Gram-positive, obligate anaerobic bacterium Clostridium tetani. Infection generally occurs through wound contamination and often involves a cut or deep puncture wound. As the infection progresses, muscle spasms develop in the jaw (thus the name "lockjaw") and elsewhere in the body.[4] Infection can be prevented by proper immunization and by post-exposure prophylaxis.[5] Generalized tetanus is the most common type of tetanus, representing about 80% of cases. The generalized form usually presents with a descending pattern. The first sign is trismus, or lockjaw, and the facial spasms called risus sardonicus, followed by stiffness of the neck, difficulty in swallowing, and rigidity of pectoral and calf muscles. Other symptoms include elevated temperature, sweating, elevated blood pressure, and episodic rapid heart rate. Spasms may occur frequently and last for several minutes with the body shaped into a characteristic form called opisthotonos. Spasms continue for up to 4 weeks, and complete recovery may take months.
Neonatal tetanus is a form of generalized tetanus that occurs in newborns. Infants who have not acquired passive immunity because the mother has never been immunized are at risk. It usually occurs through infection of the unhealed umbilical stump, particularly when the stump is cut with a non-sterile instrument. Neonatal tetanus is common in many developing countries and is responsible for about 14% (215,000) of all neonatal deaths, but is very rare in developed countries.[6]
The wound must be cleaned. Dead and infected tissue should be removed by surgical debridement. Administration of the antibiotic metronidazole decreases the number of bacteria but has no effect on the bacterial toxin. Penicillin was once used to treat tetanus, but is no longer the treatment of choice, owing to a theoretical risk of increased spasms. However, its use is recommended if metronidazole is not available. Passive immunization with human anti-tetanospasmin immunoglobulin or tetanus immunoglobulin is crucial. If specific anti-tetanospasmin immunoglobulin is not available, then normal human immunoglobulin may be given instead. All tetanus victims should be vaccinated against the disease or offered a booster shot.
Pertussis
[edit]Treatment with an effective antibiotic (erythromycin or azithromycin) shortens the infectious period but does not generally alter the outcome of the disease; however, when treatment is initiated during the catarrhal stage, symptoms may be less severe. Three macrolides (erythromycin, azithromycin and clarithromycin) are used in the U.S. for treatment of pertussis; trimethoprim-sulfamethoxazole is generally used when a macrolide is ineffective or is contraindicated. Close contacts who receive appropriate antibiotics (chemoprophylaxis) during the 7–21 day incubation period may be protected from developing symptomatic disease. Close contacts are defined as anyone coming into contact with the respiratory secretions of an infected person in the 21 days before or after the infected person's cough began.
Influenza
[edit]Influenza, commonly referred to as the flu, is an infectious disease caused by RNA viruses of the family Orthomyxoviridae (the influenza viruses), that affects birds and mammals. The most common symptoms of the disease are chills, fever, sore throat, muscle pains, severe headache, coughing, weakness/fatigue and general discomfort.[9] Sore throat, fever and coughs are the most frequent symptoms. In more serious cases, influenza causes pneumonia, which can be fatal, particularly for the young and the elderly. Although it is often confused with other influenza-like illnesses, especially the common cold, influenza is a much more severe disease than the common cold and is caused by a different type of virus.[10] Influenza may produce nausea and vomiting, particularly in children,[9] but these symptoms are more common in the unrelated gastroenteritis, which is sometimes called "stomach flu" or "24-hour flu".[11]
Typically, influenza is transmitted through the air by coughs or sneezes, creating aerosols containing the virus. Influenza can also be transmitted by direct contact with bird droppings or nasal secretions, or through contact with contaminated surfaces. Airborne aerosols have been thought to cause most infections, although which means of transmission is most important is not absolutely clear.[12] Influenza viruses can be inactivated by sunlight, disinfectants and detergents.[13][14] As the virus can be inactivated by soap, frequent hand washing reduces the risk of infection.
Influenza spreads around the world in seasonal epidemics, resulting in the deaths of between 250,000 and 500,000 people every year,[15] and millions in pandemic years. On average 41,400 people died each year in the United States between 1979 and 2001 from influenza.[16] Three influenza pandemics occurred in the 20th century and killed tens of millions of people, with each of these pandemics being caused by the appearance of a new strain of the virus in humans. Often, these new strains appear when an existing flu virus spreads to humans from other animal species, or when an existing human strain picks up new genes from a virus that usually infects birds or pigs. An avian strain named H5N1 raised the concern of a new influenza pandemic, after it emerged in Asia in the 1990s, but it has not evolved to a form that spreads easily between people.[17] In April 2009 a novel flu strain evolved that combined genes from human, pig, and bird flu, initially dubbed "swine flu" and also known as influenza A/H1N1, emerged in Mexico, the United States, and several other nations. The World Health Organization officially declared the outbreak to be a pandemic on June 11, 2009 (see 2009 flu pandemic). The WHO's declaration of a pandemic level 6 was an indication of spread, not severity, the strain actually having a lower mortality rate than common flu outbreaks.[18]
Vaccinations against influenza are usually given to people in developed countries[19] and to farmed poultry.[20] The most common human vaccine is the trivalent influenza vaccine (TIV) that contains purified and inactivated material from three viral strains. Typically, this vaccine includes material from two influenza A virus subtypes and one influenza B virus strain.[21] The TIV carries no risk of transmitting the disease, and it has very low reactivity. A vaccine formulated for one year may be ineffective in the following year, since the influenza virus evolves rapidly, and new strains quickly replace the older ones. Antiviral drugs can be used to treat influenza, with neuraminidase inhibitors being particularly effective.
Infectious Mononucleosis
[edit]Infectious Mononucleosis[22] Infectious Mononucleosis (IM) (also known as EBV Infectious Mononucleosis or Pfeiffer's Disease or Filatov's Disease[23] and colloquially as kissing disease—from its oral transmission—or as mono in North America and as glandular fever in other English-speaking countries) is an infectious, very widespread viral disease caused by the Epstein-Barr virus (EBV), one type of herpes virus, to which more than 90% of adults have been exposed.[24] Most people are exposed to the virus as children, when the disease produces no noticeable symptoms or only flu-like symptoms. In underdeveloped countries, people are exposed to the virus in early childhood more often than in developed countries, which is why the disease in its observable form is more common in developed countries. It is most common among adolescents and young adults. [25]
Especially in adolescents and young adults, the disease is characterized by fever, sore throat and fatigue, along with several other possible signs and symptoms. It is primarily diagnosed by observation of symptoms, but suspicion can be confirmed by several diagnostic tests.
The syndrome was described as an infectious process by Nil Filatov[26] in 1887 and independently by Emil Pfeiffer[27] in 1889.[23]
Retrieved on 7 Mars, 2009</ref>[28][29]]] The classical symptoms of mononucleosis are a sore throat, fever, fatigue, weight loss, malaise, pharyngeal inflammation, petechiae and loss of appetite. Common signs include lymphadenopathy (enlarged lymph nodes), splenomegaly (enlarged spleen), hepatitis (refers to inflammation of hepatocytes - cells in the liver) and hemolysis (the bursting of red blood cells). Older adults are less likely to have a sore throat or lymphadenopathy, but are instead more likely to present with hepatomegaly (enlargement of the liver) and jaundice. Rarer signs and symptoms include thrombocytopenia (lower levels of platelets), with or without pancytopenia (lower levels of all types of blood cells), splenic rupture, splenic hemorrhage, upper airway obstruction, pericarditis and pneumonitis. Another rare manifestation of mononucleosis is erythema multiforme.[30][31]
Ebola hemorrhagic fever
[edit]Ebola is the virus Ebolavirus (EBOV), a viral genus, and the disease Ebola hemorrhagic fever (EHF), a viral hemorrhagic fever (VHF). The virus is named after the Ebola River Valley in the Democratic Republic of the Congo (formerly Zaire), which is near the site of the first recognized outbreak, a mission hospital run by Flemish nuns, in 1976.[33] There are four recognized species within the ebolavirus genus, which have a number of specific strains.[34] The Zaire virus is the type species, which is also the first discovered and the most lethal. Electron micrographs show long filaments, characteristic of the Filoviridae viral family. The virus interferes with the endothelial cells lining the interior surface of blood vessels and with coagulation. As the blood vessel walls become damaged and the platelets are unable to coagulate, patients succumb to hypovolemic shock. Ebola is transmitted through bodily fluids, while conjunctiva exposure may also lead to transmission. Ebola first emerged in 1976 in Zaire. However, it remained largely obscure until 1989 when several widely publicized outbreaks among monkeys in the United States occurred.
Classification:
The genera Ebolavirus and Marburgvirus was originally classified as the species of the now-obsolete Filovirus genus. In March 1998, the Vertebrate Virus Subcommittee proposed in the International Committee on Taxonomy of Viruses (ICTV) to change the Filovirus genus to the Filoviridae family with two specific genera: Ebola-like viruses and Marburg-like viruses. This proposal was implemented in Washington, D.C. as of April ,2001 and in Paris as of July 2002. In 2000, another proposal was made in Washington, D.C. to change the "-like viruses" to "-virus" resulting in today's Ebolavirus and Marburgvirus.[35]
Rates of genetic change are one hundred times slower than Influenza A in humans, but on the same magnitude of that of Hepatitis B. Using these rates, the Ebolavirus and Marburgvirus are estimated to have diverged several thousand years ago.[36]
- Zaire virus (ZEBOV)
- The Zaire virus, formerly named Zaire Ebola Virus, has the highest case-fatality rate, up to 90% in some epidemics, with an average case fatality rate of approximately 83% over 27 years. There have been more outbreaks of Zaire ebolavirus than any other species. The first outbreak took place on 26 August 1976 in Yambuku. Mabalo Lokela, a 44-year-old schoolteacher, became the first recorded case. The symptoms resembled malaria, and subsequent patients received quinine. The initial transmission was believed to be due to reuse of the needle for Lokela's injection without sterilization. Subsequent transmission was also due to lack of barrier nursing and the traditional burial preparation method, which involves washing and gastrointestinal tract cleansing.[37]
- Sudan ebolavirus (SEBOV)
- The virus was the second species of Ebola emerging simultaneous with the Zaire virus. It was believed to have originated amongst cotton factory workers in Nzara, Sudan, with the first case reported as a worker exposed to a potential natural reservoir. Scientists tested all animals and insects in response to this; however, none tested positive for the virus. The carrier is still unknown. The lack of barrier nursing facilitated the spread of the disease. The most recent outbreak occurred in May 2004. 20 confirmed cases were reported in Yambio County, Sudan, with five deaths resulting. The average fatality rates for were 54% in 1976, 68% in 1979, and 53% in 2000 and 2001.
- Reston ebolavirus (REBOV)
- Discovered during an outbreak of Simian hemorrhagic fever virus (SHFV) in crab-eating macaques from Hazleton Laboratories (now Covance) in 1989. Since the initial outbreak in Reston, Virginia, it has emerged in the Philippines, Siena Italy, Texas,[38] and among pigs in the Philippines.[39] Despite its status as a Level-4 organism, it is non-pathogenic to humans however hazardous in monkeys.[40]
- Cote d'Ivoire ebolavirus (CIEBOV)
- Also referred to as Ivory Coast ebolavirus and Tai ebolavirus, it was first discovered among chimpanzees from the Tai Forest in Côte d'Ivoire, Africa on 1 November 1994. Necropsies showed blood within the heart to be brown, no obvious marks were seen on the organs, and one necropsy displayed lungs filled with blood. Studies of tissues taken from the chimpanzees showed results similar to human cases during the 1976 Ebola outbreaks in Zaire and Sudan. As more dead chimpanzees were discovered, with many testing positive to Ebola using molecular techniques. The source of contamination was believed to be the meat of infected Western Red Colobus monkeys, upon which the chimpanzees preyed. One of the scientists performing the necropsies on the infected chimpanzees contracted Ebola. She developed symptoms similar to those of dengue fever approximately a week after the necropsy, and was transported to Switzerland for treatment. She was discharged from hospital after two weeks and had fully recovered six weeks after the infection.[41]
- Bundibugyo ebolavirus
- On November 24, 2007, the Uganda Ministry of Health confirmed an outbreak of Ebola in the Bundibugyo District. After confirmation of samples tested by the United States National Reference Laboratories and the CDC, the World Health Organization confirmed the presence of the new species. On 20 February 2008, the Uganda Ministry officially announced the end of the epidemic in Bundibugyo with the last infected person discharged on 8 January 2008.[42] Ugandan officials confirmed a total of 149 cases of this new Ebola species, with 37 deaths attributed to the strain (24.83%).[43]
Virology, Structure, and Genome
Each virion contains one molecule of linear, single-stranded, negative-sense RNA, 18,959 to 18,961 nucleotides in length. The 3′ terminus is not polyadenylated and the 5′ end is not capped. It was found that 472 nucleotides from the 3' end and 731 nucleotides from the 5' end are sufficient for replication.[44] It codes for seven structural proteins and one non-structural protein. The gene order is 3′ - leader - NP - VP35 - VP40 - GP/sGP - VP30 - VP24 - L - trailer - 5′; with the leader and trailer being non-transcribed regions, which carry important signals to control transcription, replication, and packaging of the viral genomes into new virions. The genomic material by itself is not infectious, because viral proteins, among them the RNA-dependent RNA polymerase, are necessary to transcribe the viral genome into mRNAs, as well as for replication of the viral genome.
Replication:
Being acellular, viruses do not grow through cell division; instead, they use the machinery and metabolism of a host cell to produce multiple copies of themselves, and they assemble in the cell.[45]
- The virus attaches to host receptors through the glycoprotein (GP) surface peplomer and is endocytosed into vesicles in the host cell
- Viral membrane fuses with vesicle membrane, nucleocapsid is released into the cytoplasm
- Encapsidated, negative-sense genomic ssRNA is used as a template for the synthesis (3' - 5') of polyadenylated, monocistronic mRNAs
- Using the host cell's machinery translation of the mRNA into viral proteins occurs
- Viral proteins are processed, glycoprotein precursor (GP0) is cleaved to GP1 and GP2, which are heavily glycosylated. These two molecules assemble, first into heterodimers, and then into trimers to give the surface peplomers. Secreted glycoprotein (sGP) precursor is cleaved to sGP and delta peptide, both of which are released from the cell.
- As viral protein levels rise, a switch occurs from translation to replication. Using the negative-sense genomic RNA as a template, a complementary +ssRNA is synthesized; this is then used as a template for the synthesis of new genomic (-)ssRNA, which is rapidly encapsidated.
- The newly-formed nucleocapsides and envelope proteins associate at the host cell's plasma membrane; budding occurs.
Pathogenesis of ebola hemorrhagic fever:
Endothelial cells, mononuclear phagocytes, and hepatocytes are the main targets of infection. After infection, in a secreted glycoprotein (sGP) the Ebola virus glycoprotein (GP) is synthesized. Ebola replication overwhelms protein synthesis of infected cells and host immune defenses. The GP forms a trimeric complex, which binds the virus to the endothelial cells lining the interior surface of blood vessels. The sGP forms a dimeric protein which interferes with neutrophils, which are a type of white blood cell, signaling which allows the virus to evade the immune system by inhibiting early steps of neutrophil activation. The presence of viral particles and cell damage resulting from budding causes the release of cytokines (specifically TNF-α, IL-6, IL-8, etc), which are the signaling molecules for fever and inflammation. The cytopathic effect, from infection in the endothelial cells, results in a loss of vascular integrity. This loss in vascular integrity is furthered with synthesis of GP, which reduces specific integrins responsible for cell adhesion to the inter-cellular structure, and damage to the liver, which leads to coagulopathy. Without vascular integrity and effective coagulation, blood quickly leaks through the blood vessel leading to hypovolemic shock.[46]
Epidemiology of ebola hemorrhagic fever: Natural reservoirs of Ebola virus:
Between 1976 and 1998, from 30,000 mammals, birds, reptiles, amphibians, and arthropods sampled from outbreak regions, no Ebolavirus was detected apart from some genetic material found in six rodents (Mus setulosus and Praomys) and one shrew (Sylvisorex ollula) collected from the Central African Republic.[47][48] The virus was detected in the carcasses of gorillas, chimpanzees, and duikers during outbreaks in 2001 and 2003, which later became the source of human infections. However, the high mortality from infection in these species makes them unlikely as a natural reservoir.[47]
Plants, arthropods, and birds have also been considered as possible reservoirs, however, bats are considered the most likely candidate.[49] Bats were known to reside in the cotton factory in which the index cases for the 1976 and 1979 outbreaks were employed, and they have also been implicated in Marburg infections in 1975 and 1980.[47] Of 24 plant species and 19 vertebrate species experimentally inoculated with Ebolavirus, only bats became infected.[50] The absence of clinical signs in these bats is characteristic of a reservoir species. In a 2002–2003 survey of 1,030 animals which included 679 bats from Gabon and the Republic of the Congo, 13 fruit bats were found to contain Ebolavirus RNA.[51] As of 2005, three fruit bat species (Hypsignathus monstrosus, Epomops franqueti, and Myonycteris torquata) have been identified as carrying the virus while remaining asymptomatic. They are believed to be a natural host species, or reservoir, of the virus.[52]
Reston ebolavirus—unlike its African counterparts—is non-pathogenic in humans. The high mortality among monkeys and its recent emergence in swine, makes them an unlikely natural reservoirs.[53]
Transmission of ebola hemorrhagic fever:
Bats drop partially eaten fruits and pulp, terrestrial mammals such as gorillas and duikers feed on these fallen fruits. This chain of events forms a possible indirect means of transmission from the natural host to animal populations, which have led to research towards viral shedding in the saliva of bats. Fruit production, animal behavior, and other factors vary at different times and places which may trigger outbreaks among animal populations.[54] Transmission between natural reservoirs and humans are rare, and outbreaks are usually traceable to a single index case where an individual has handled the carcass of gorilla, chimpanzee, or duiker.[55] The virus then spreads person-to-person, especially within families, hospitals, and during some mortuary rituals where contact among individuals becomes more likely.[56]
The virus has been confirmed to be transmitted through body fluids. Transmission through oral exposure and through conjunctiva exposure is likely,[57] which have been confirmed in non-human primates.[58] Filoviruses are not naturally transmitted by aerosol. They are, however, highly infectious as breathable 0.8-1.2 micron droplets in laboratory conditions;[59] because of this potential route of infection, these viruses have been classified as Category A biological weapons.[60]
All epidemics of Ebola have occurred in sub-optimal hospital conditions, where practices of basic hygiene and sanitation are often either luxuries or unknown to caretakers and where disposable needles and autoclaves are unavailable or too expensive. In modern hospitals with disposable needles and knowledge of basic hygiene and barrier nursing techniques, Ebola has never spread on a large scale. In isolated settings such as a quarantined hospital or a remote village, most victims are infected shortly after the first case of infection is present. The quick onset of symptoms from the time the disease becomes contagious in an individual makes it easy to identify sick individuals and limits an individual's ability to spread the disease by traveling. Because bodies of the deceased are still infectious, some doctors had to take measures to properly dispose dead bodies in a safe manner despite local traditional burial rituals.[61]
Prevalence of Ebola hemorrhagic fever:
Outbreaks of Ebola, with the exception of Reston ebolavirus, have mainly been restricted to Africa. The virus often consumes the population, governments and individuals quickly respond to quarantine the area, and the lack of roads and transportation—helps to contain the outbreak.[38]
- Zaire virus first emerged in an outbreak among human populations in 1976 in Zaire (now Democratic Republic of the Congo) with no further recognized cases until 1994. Since then it has occurred again in the Democratic Republic of the Congo, Republic of the Congo, and Gabon. There have been two contained cases in South Africa.
- Sudan ebolavirus emerged in a simultaneous outbreak with the Zaire virus in 1976 in Sudan. It appeared again in another outbreak in 1979. No further cases were recognized until a 2000 outbreak in Uganda and 2004 outbreak in Sudan. There has been one confirmed accidental incidence in 1976 in England.
- Reston ebolavirus was first recognized among monkeys in 1989 in the Reston, Virginia and again in Alice, Texas in the United States, both were traced to the Philippines. In 1994 it was recognized in cases among monkeys in an import facility in Italy. In 2008 cases of infection among pigs were recognized in the Philippines.
- Ivory Coast ebolavirus was first recognized in 1994 after a scientist became ill after conducting an autopsy on a wild chimpanzee in the Tai Forest, Côte d'Ivoire.
- Bundibugyo ebolavirus was first recognized in 2007 in an outbreak in Bundibugyo District, Uganda.
Clinical aspects of Ebola hemorrhagic fever: Prevention of Ebola hemorrhagic fever: In the early stages, Ebola may not be highly contagious. Contact with someone in early stages may not even transmit the disease. As the illness progresses, bodily fluids from diarrhea, vomiting, and bleeding represent a hazard. Due to lack of proper equipment and hygienic practices, large-scale epidemics occur mostly in poor, isolated areas without modern hospitals or well-educated medical staff. Many areas where the infectious reservoir exists have just these characteristics. In such environments, all that can be done is to immediately cease all needle-sharing or use without adequate sterilization procedures, isolate patients, and observe strict barrier nursing procedures with the use of a medical rated disposable face mask, gloves, goggles, and a gown at all times. This should be strictly enforced for all medical personnel and visitors.[62]
Vaccines have successfully protected non-human primates, however the six months needed to complete immunization made it impractical in an epidemic. To resolve this, in 2003 a vaccine using an adenoviral (ADV) vector carrying the Ebola spike protein was tested on crab-eating macaques. The monkeys were challenged with the virus twenty-eight days later, and remained resistant.[63] In 2005 a vaccine based on attenuated recombinant vesicular stomatitis virus (VSV) vector carrying either the Ebola glycoprotein or Marburg glycoprotein successfully protected non-human primates,[64] opening clinical trials in humans.[65] By October the study completed the first human trial giving three vaccinations over three months showing capability of safely inducing an immune response. Individuals were followed for a year, and in 2006 a study testing a faster-acting, single shot vaccine began. This study was completed in 2008.[66]
Symptoms of Ebola hemorrhagic fever:
The incubation period ranges from 2–21 days, although it is generally 5–18 days,[67] although Bundibugyo ebolavirus may be more than twice as long at 42 days.[68] Illness is characterized by the rapid onset of fever, malaise, muscle pain, headache, and the inflammation of the pharynx. Six days following vomiting and bloody diarrhea, individuals may develop maculopapular rash with bleeding at needle sites and bodily orifices.[69]
Reston ebolavirus is non-pathogenic to humans and individuals often do not show any symptoms, although it is fatal in monkeys. There is only one known case of Ivory Coast ebolavirus. There has been only one outbreak of Bundibugyo ebolavirus. Zaire virus, then Sudan ebolavirus, are the most common. Symptoms include: abdominal pain (60-80%), fever (90%-100%), headache (40%-90%), bloody vomit (10%-40%), Maculopapular rash (5%-20%), malaise (75%-85%), joint and muscle pain (40%-80%), inflammation of the pharynx (20%-40%),[70] blood fails to clot (71%-78%), chest pain (SEBOV only 83%), CNS involvement (rare), dry and sore throat (63%), hemorrhagic diathesis (71%-78%), hiccups (15%), non-bloody diarrhea (81%), vomiting (59%).[71] Purpura, petechia, sclerotic arterioles, and low blood-pressure are characteristic as the disease progresses.
Marburg hemorrhagic fever
[edit]
Marburg virus or simply Marburg is the common name for the the genus of viruses Marburgvirus, which contains one species, Lake Victoria marburgvirus. The virus causes the disease Marburg Hemorrhagic Fever (MHF), also referred to as Marburg Virus Disease, and previously also known as green monkey disease due to its primate origin. Marburg originated in Central and East Africa, and infects both human and nonhuman primates. The Marburg Virus is in the same taxonomic family as Ebola, and both are identical structurally although they elicit different antibodies.
Etymology:
The genera Marburgvirus and Ebolavirus were originally classified as the species of the now nonexistent Filovirus genus. In March 1998, the Vertebrate Virus Subcommittee proposed to the International Committee on Taxonomy of Viruses (ICTV) to change the Filovirus genus to Filovirus family with two specific genera: Ebola-like viruses and Marburg-like viruses. This proposal was implemented in Washington DC as of April 2001 and in Paris as of July 2002. In 2000, another proposal was made in Washington DC to change the "-like viruses" to "-virus" (e.g. Ebolavirus, Marburgvirus) in addition to renaming the only species in the Marburgvirus genus from Marburg virus to Lake Victoria Marburgvirus.[73]
The item "Marburg" was named after the location of the first outbreak in 1967 in Marburg, Germany.
Virology and Structure:
The viral structure is typical of filoviruses, with long threadlike particles which have a consistent diameter but vary greatly in length from an average of 800 to 14,000 nanometers (nm), with peak infectious activity at about 790 nm. Virions (viral particles) contain seven known structural proteins. While nearly identical to Ebola virus in structure, Marburg virus is antigenically distinct from Ebola virus; in other words, it triggers different antibodies in infected organisms. It was the first filovirus to be identified.
Genome:
Marburg contains a single molecule of linear negative-sense, 19100 nucleotides long, single-stranded RNA.[74]
Natural reservoir:
In September 2007, New Scientist magazine reported that the virus has been found in cave-dwelling African fruit bats in Gabon, the first time the virus has been found outside primates. The virus has now also been confirmed in bats in a Uganda mine[3] after two miners contracted Marburg in August 2007. Ebola antibodies (a close relative to Marburg) were found in three species of fruit bats in 2005. Marburg antibodies have been found in healthy bats, suggesting that the bats had been previously infected. Although no one has yet found complete live viruses from a bat, the team suggest that "[I] think we can be sure that these fruit bats are the reservoir of Marburg virus".[75]
The same techniques used to identify those genes were also used to identify Marburg genes found in Egyptian fruit bats, Rousettus aegyptiacus.[76]
Epidemiology and Prevalence:
Outbreaks of Marburg are centered in Africa, where the natural reservoir is believed to be located.
Transmission of marburg hemorrhagic fever:
The disease is spread through bodily fluids, including blood, excrement, saliva, and vomit. Early symptoms are often non-specific, and usually include fever, headache and myalgia after an incubation period of three to nine days. After five days, a maculopapular rash is often present on the trunk. Later-stage Marburg infection is acute and can include jaundice, pancreatitis, weight loss, delirium and neuropsychiatric symptoms, haemorrhaging, hypovolemic shock and multi-organ dysfunction, with liver failure most common. Accounts of external haemorrhaging from bodily orifices are pervasive in popular references to the disease but are in fact rare. Time course varies but symptoms usually last for one to three weeks until the disease either resolves or kills the infected host. The fatality rate is from 23% to over 90%.[77][78]
Medical aspects of marburg hemorrhagic fever:
Caregivers require barrier infection control measures including double gloves, impermeable gowns, face shields, eye protection, leg and shoe coverings.
Marburg is a biosafety level-four agent, and thus requiring the highest level of precautions.[79]
A few research groups are working on drugs and vaccines to fight the virus. In 1998, a group at the United States Army Medical Research Institute of Infectious Diseases (USAMRIID) published the first peer reviewed article detailing the development of the first experimental Marburg virus vaccine demonstrated to completely protect animals from lethal Marburg virus infection[80] Following, in 2002, Genphar, a company doing research for the United States Army's biodefense program, announced that an experimental vaccine protected animals from a high dose of Marburg virus. The tests were conducted by the United States Army Medical Research Institute of Infectious Diseases (USAMRIID). According to the company, all animals in the control group died within days whereas all animals that received the regular dosage of the vaccine were fully protected.
In June 2005 scientists at Canada's National Microbiology Laboratory announced that they had also developed vaccines for both Marburg and Ebola that showed significant promise in primate testing. Studies on mice also suggested that the vaccine might be an effective treatment for the disease if it is administered shortly after a patient is infected. To make the vaccines the scientists fused a surface protein from the viruses they hope to protect against onto an animal virus - vesicular stomatitis - which is thought to be of no threat to humans.[81] In the rhesus macaque monkey model of the disease, the vaccine is effective even when given after infection with the virus.[82]
Symptoms of marburg hemorrhagic fever:
Many of the symptoms of Marburg haemorrhagic fever are similar to those of other infectious diseases, such as malaria or typhoid, but are most similar to those of Ebola strains.
Diagnosing marburg hemorrhagic fever:
Diagnosis of Marburg is similar to Ebola using the Enzyme-Linked ImmunoSorbent Assay (ELISA) test.[83]
Prognosis of marburg hemorrhagic fever:
If a patient survives, recovery is usually prompt and complete, though it may be prolonged in some cases, with inflammation or secondary infection of various organs, including: orchitis (testicles), hepatitis (liver), transverse myelitis (spinal cord), uveitis (eyes), and parotitis (salivary glands)[citation needed]. Recovered patients often have little or no memory of being sick, though only 10-40% survive.
Treatment:
There is no specific antiviral therapy indicated for treating Marburg, and hospital care is usually supportive in nature. Hypotension and shock may require early administration of vasopressors and haemodynamic monitoring with attention to fluid and electrolyte balance, circulatory volume, and blood pressure. Viral haemorrhagic fever (VHF) patients tend to respond poorly to fluid infusions and may develop pulmonary edema.
Streptococcal pharyngitis
[edit]Streptococcal pharyngitis or streptococcal sore throat (known colloquially as strep throat in American English) is a form of group A streptococcal infection[85] that affects the pharynx and possibly the larynx and tonsils. It is a very contagious infection, spread by close contact with an infected individual, which can lead to various other complications if not swiftly treated. Antibiotics can help reduce contagiousness.
Signs and symptoms of streptococcal pharyngitis: Streptococcal pharyngitis usually appears suddenly with severe sore throat pain that may make talking or swallowing painful.
Signs and symptoms may include
- Inflamed tonsils
- White spots on the tonsils[86]
- Difficulty swallowing (dysphagia)
- Tender cervical lymphadenopathy
- Bumps, bruises, inflamation, or swelling; (goose eggs), on the right, or uncommonly left side of neck.
- Fever
- Headache (often prior to other symptoms)
- Malaise, general discomfort, feeling ill or uneasy
- Halitosis
- Abdominal pain, nausea and vomiting[87]
- Rash[88]
- Hives
- Chills
- Loss of appetite
- Ear pain
- Peeling of skin on hands and feet
- Ears locking up
Additional symptoms such as sinusitis, vaginitis, or impetigo may be present if the strep bacteria infects both the throat and a secondary location. For additional information on non-pharynx symptoms, see Group A Streptococcal (GAS) Infection.
Diagnosis of streptococcal pharyngitis: There are several causes for pharyngitis, not just streptococcus bacteria. Productive coughing, nasal discharge, and red, irritated eyes in addition to fever and sore throat are more indicative of a viral sore throat than of strep throat, though a co-infection with a virus is possible and may explain the presence of these additional symptoms. A rapid strep test (also called rapid antigen detection testing or RADT) or a throat culture may be undertaken to clarify diagnosis. The rapid strep test is quicker but less sensitive and specific than a throat culture developed on a blood agar plate.[89] Positive tests in association with symptoms establish a positive diagnosis, which can be treated with antibiotics.[89] Asymptomatic patients should not be routinely tested with a throat culture because a certain percentage of the population persistently "carries" strep throat.[89]
Pneumonia
[edit]Pneumonia is an abnormal inflammatory condition of the lung.[91] It is often characterized as including inflammation of the parenchyma of the lung (that is, the alveoli) and abnormal alveolar filling with fluid (consolidation and exudation).[92]
The alveoli are microscopic air-filled sacs in the lungs responsible for gas exchange. Pneumonia can result from a variety of causes, including infection with bacteria, viruses, fungi, or parasites, and chemical or physical injury to the lungs. Its cause may also be officially described as idiopathic—that is, unknown—when infectious causes have been excluded.
Typical symptoms associated with pneumonia include cough, chest pain, fever, and difficulty in breathing. Diagnostic tools include x-rays and examination of the sputum. Treatment depends on the cause of pneumonia; bacterial pneumonia is treated with antibiotics.
Pneumonia is a common illness which occurs in all age groups, and is a leading cause of death among the elderly and people who are chronically and terminally ill. Additionally, it is the leading cause of death in children under five years old worldwide.[93] Vaccines to prevent certain types of pneumonia are available. The prognosis depends on the type of pneumonia, the appropriate treatment, any complications, and the person's underlying health.
Classification of Pneumonia: Pneumonias can be classified in several ways. Pathologists originally classified them according to the anatomic changes that were found in the lungs during autopsies. As more became known about the microorganisms causing pneumonia, a microbiologic classification arose, and with the advent of x-rays, a radiological classification. Another important system of classification is the combined clinical classification, which combines factors such as age, risk factors for certain microorganisms, the presence of underlying lung disease and underlying systemic disease, and whether the person has recently been hospitalized.
Early classification schemes: Initial descriptions of pneumonia focused on the anatomic or pathologic appearance of the lung, either by direct inspection at autopsy or by its appearance under a microscope.
- A lobar pneumonia is an infection that only involves a single lobe, or section, of a lung. Lobar pneumonia is often due to Streptococcus pneumoniae (though Klebsiella pneumoniae is also possible.)[94]
- Multilobar pneumonia involves more than one lobe, and it often causes a more severe illness.
- Bronchial pneumonia affects the lungs in patches around the tubes (bronchi or bronchioles).
- Interstitial pneumonia involves the areas in between the alveoli, and it may be called "interstitial pneumonitis." It is more likely to be caused by viruses or by atypical bacteria.
The discovery of x-rays made it possible to determine the anatomic type of pneumonia without direct examination of the lungs at autopsy and led to the development of a radiological classification. Early investigators distinguished between typical lobar pneumonia and atypical (e.g. Chlamydophila) or viral pneumonia using the location, distribution, and appearance of the opacities they saw on chest x-rays. Certain x-ray findings can be used to help predict the course of illness, although it is not possible to clearly determine the microbiologic cause of a pneumonia with x-rays alone.
With the advent of modern microbiology, classification based upon the causative microorganism became possible. Determining which microorganism is causing an individual's pneumonia is an important step in deciding treatment type and length. Sputum cultures, blood cultures, tests on respiratory secretions, and specific blood tests are used to determine the microbiologic classification. Because such laboratory testing typically takes several days, microbiologic classification is usually not possible at the time of initial diagnosis.
Combined clinical classification: Traditionally, clinicians have classified pneumonia by clinical characteristics, dividing them into "acute" (less than three weeks duration) and "chronic" pneumonias. This is useful because chronic pneumonias tend to be either non-infectious, or mycobacterial, fungal, or mixed bacterial infections caused by airway obstruction. Acute pneumonias are further divided into the classic bacterial bronchopneumonias (such as Streptococcus pneumoniae), the atypical pneumonias (such as the interstitial pneumonitis of Mycoplasma pneumoniae or Chlamydia pneumoniae), and the aspiration pneumonia syndromes.
Chronic pneumonias, on the other hand, mainly include those of Nocardia, Actinomyces and Blastomyces dermatitidis, as well as the granulomatous pneumonias (Mycobacterium tuberculosis and atypical mycobacteria, Histoplasma capsulatum and Coccidioides immitis).[95]
The combined clinical classification, now the most commonly used classification scheme, attempts to identify a person's risk factors when he or she first comes to medical attention. The advantage of this classification scheme over previous systems is that it can help guide the selection of appropriate initial treatments even before the microbiologic cause of the pneumonia is known. There are two broad categories of pneumonia in this scheme: community-acquired pneumonia and hospital-acquired pneumonia. A recently introduced type of healthcare-associated pneumonia (in patients living outside the hospital who have recently been in close contact with the health care system) lies between these two categories.
Community-acquired Pneumonia:
Community-acquired pneumonia (CAP) is infectious pneumonia in a person who has not recently been hospitalized. CAP is the most common type of pneumonia. The most common causes of CAP vary depending on a person's age, but they include Streptococcus pneumoniae, viruses, the atypical bacteria, and Haemophilus influenzae. Overall, Streptococcus pneumoniae is the most common cause of community-acquired pneumonia worldwide. Gram-negative bacteria cause CAP in certain at-risk populations. CAP is the fourth most common cause of death in the United Kingdom and the sixth in the United States. The term "walking pneumonia" has been used to describe a type of community-acquired pneumonia of less severity (because the sufferer can continue to "walk" rather than require hospitalization).[96] Walking pneumonia is usually caused by the atypical bacterium, Mycoplasma pneumoniae.[97]
Septicemia
[edit]Sepsis is a serious medical condition that is characterized by a whole-body inflammatory state (called a systemic inflammatory response syndrome or SIRS) and the presence of a known or suspected infection.[99][100] The body may develop this inflammatory response to microbes in the blood, urine, lungs, skin, or other tissues. A lay term for sepsis is blood poisoning, more aptly applied to septicemia, below.
Septicemia (also septicaemia or septicæmia [sep⋅ti⋅cæ⋅mi⋅a][101], or erroneously septasemia and septisema) is a related but deprecated (formerly sanctioned) medical term referring to the presence of pathogenic organisms in the bloodstream, leading to sepsis.[102] The term has not been sharply defined. It has been inconsistently used in the past by medical professionals, for example as a synonym of bacteremia, causing some confusion. The present medical consensus is therefore that the term "septicemia" is problematic and should be avoided.[100]
Sepsis is usually treated in the intensive care unit with intravenous fluids and antibiotics. If fluid replacement is insufficient to maintain blood pressure, specific vasopressor medications can be used. Artificial ventilation and dialysis may be needed to support the function of the lungs and kidneys, respectively. To guide therapy, a central venous catheter and an arterial catheter may be placed. Sepsis patients require preventive measures for deep vein thrombosis, stress ulcers and pressure ulcers, unless other conditions prevent this. Some patients might benefit from tight control of blood sugar levels with insulin (targeting stress hyperglycemia), low-dose corticosteroids or activated drotrecogin alfa (recombinant protein C).[103]
Terminology: Severe sepsis occurs when sepsis leads to organ dysfunction, low blood pressure (hypotension), or insufficient blood flow (hypoperfusion) to one or more organs (causing, for example, lactic acidosis, decreased urine production, or altered mental status). Sepsis can lead to septic shock, multiple organ dysfunction syndrome (formerly known as multiple organ failure), and death. Organ dysfunction results from sepsis-induced hypotension (< 90 mmHg or a reduction of ≥ 40 mmHg from baseline) and diffuse intravascular coagulation, among other things.
Bacteremia is the presence of viable bacteria in the bloodstream. Likewise, the terms viremia and fungemia simply refer to viruses and fungi in the bloodstream. These terms say nothing about the consequences this has on the body. For example, bacteria can be introduced into the bloodstream during toothbrushing.[104] This form of bacteremia almost never causes problems in normal individuals. However, bacteremia associated with certain dental procedures can cause bacterial infection of the heart valves (known as endocarditis) in high-risk patients.[105] Conversely, a systemic inflammatory response syndrome can occur in patients without the presence of infection, for example in those with burns, polytrauma, or the initial state in pancreatitis and chemical pneumonitis.[100]
Signs and symptoms of septicemia: In addition to symptoms related to the provoking infection, sepsis is characterized by evidence of acute inflammation present throughout the entire body, and is, therefore, frequently associated with fever and elevated white blood cell count (leukocytosis) or low white blood cell count and lower-than-average temperature, and vomiting [citation needed]. The modern concept of sepsis is that the host's immune response to the infection causes most of the symptoms of sepsis, resulting in hemodynamic consequences and damage to organs. This host response has been termed systemic inflammatory response syndrome (SIRS) and is characterized by hemodynamic compromise and resultant metabolic derangement. Outward physical symptoms of this response frequently include a high heart rate (above 100 beats per minute), high respiratory rate (above 20 breaths per minute), elevated WBC count (above 12,000) and elevated or lowered body temperature (under 36 °C or over 38 °C). Sepsis is differentiated from SIRS by the presence of a known pathogen. For example SIRS and a positive blood culture for a pathogen indicates the presence of sepsis. Without a known infection, it's not possible to classify the above symptoms as sepsis, only SIRS.
This immunological response causes widespread activation of acute-phase proteins, affecting the complement system and the coagulation pathways, which then cause damage to the vasculature as well as to the organs. Various neuroendocrine counter-regulatory systems are then activated as well, often compounding the problem. Even with immediate and aggressive treatment, this may progress to multiple organ dysfunction syndrome and eventually death.
Malaria
[edit]Malaria is a mosquito-borne infectious disease caused by a eukaryotic protist of the genus Plasmodium. It is widespread in tropical and subtropical regions, including parts of the Americas, Asia, and Africa. Each year, there are approximately 350–500 million cases of malaria,[107] killing between one and three million people, the majority of whom are young children in sub-Saharan Africa.[108] Ninety percent of malaria-related deaths occur in sub-Saharan Africa. Malaria is commonly associated with poverty, but is also a cause of poverty[109] and a major hindrance to economic development.
Five species of the plasmodium parasite can infect humans; the most serious forms of the disease are caused by Plasmodium falciparum. Malaria caused by Plasmodium vivax, Plasmodium ovale and Plasmodium malariae causes milder disease in humans that is not generally fatal. A fifth species, Plasmodium knowlesi, is a zoonosis that causes malaria in macaques but can also infect humans.[110][111]
Malaria is naturally transmitted by the bite of a female Anopheles mosquito. When a mosquito bites an infected person, a small amount of blood is taken, which contains malaria parasites. These develop within the mosquito, and about one week later, when the mosquito takes its next blood meal, the parasites are injected with the mosquito's saliva into the person being bitten. After a period of between two weeks and several months (occasionally years) spent in the liver, the malaria parasites start to multiply within red blood cells, causing symptoms that include fever and headache. In severe cases, the disease worsens, leading to coma and death.
A wide variety of antimalarial drugs are available to treat malaria. In the last 5 years, treatment of P. falciparum infections in endemic countries has been transformed by the use of combinations of drugs containing an artemisinin derivative. Severe malaria is treated with intravenous or intramuscular quinine or, increasingly, the artemisinin derivative artesunate[112]. Several drugs are also available to prevent malaria in travellers to malaria-endemic countries (prophylaxis). Resistance has developed to several antimalarial drugs, most notably chloroquine[113].
Malaria transmission can be reduced by preventing mosquito bites by distribution of inexpensive mosquito nets and insect repellents, or by mosquito-control measures such as spraying insecticides inside houses and draining standing water where mosquitoes lay their eggs.
Although many are under development, the challenge of producing a widely available vaccine that provides a high level of protection for a sustained period is still to be met[114].
Dengue fever
[edit]
Dengue fever (pronounced English pronunciation: /ˈdɛŋɡeɪ/, English pronunciation: /ˈdɛŋɡiː/) and dengue hemorrhagic fever (DHF) are acute febrile diseases which occur in the tropics, can be life-threatening, and are caused by four closely related virus serotypes of the genus Flavivirus, family Flaviviridae.[115] It is also known as breakbone fever. It occurs widely in the tropics, including northern Argentina, northern Australia, the entirety of Bangladesh, Barbados, Bolivia[116], Belize, Brazil, Cambodia, Costa Rica, Dominican Republic, El Salvador, Guatemala, Guyana, Honduras, India, Indonesia, Jamaica, Laos, Malaysia, Mexico, Micronesia, Panama, Paraguay[117], Philippines, Puerto Rico, Samoa[118], Singapore, Sri Lanka, Suriname, Taiwan, Thailand, Trinidad, Venezuela and Vietnam, and increasingly in southern China[119]. Unlike malaria, dengue is just as prevalent in the urban districts of its range as in rural areas. Each serotype is sufficiently different that there is no cross-protection and epidemics caused by multiple serotypes (hyperendemicity) can occur. Dengue is transmitted to humans by the Aedes aegypti or more rarely the Aedes albopictus mosquito, which feed during the day.[120]
The WHO says some 2.5 billion people, two fifths of the world's population, are now at risk from dengue and estimates that there may be 50 million cases of dengue infection worldwide every year. The disease is now endemic in more than 100 countries.[121]
Signs and symptoms of Dengue fever: The disease manifests as a sudden onset of severe headache, muscle and joint pains (myalgias and arthralgias—severe pain that gives it the nickname break-bone fever or bonecrusher disease), fever, and rash.[122] The dengue rash is characteristically bright red petechiae and usually appears first on the lower limbs and the chest; in some patients, it spreads to cover most of the body. There may also be gastritis with some combination of associated abdominal pain, nausea, vomiting, or diarrhea.
Some cases develop much milder symptoms which can be misdiagnosed as influenza or other viral infection when no rash is present. Thus travelers from tropical areas may pass on dengue inadvertently, having not been properly diagnosed at the height of their illness. Patients with dengue can pass on the infection only through mosquitoes or blood products and only while they are still febrile. The classic dengue fever lasts about two to seven days, with a smaller peak of fever at the trailing end of the disease (the so-called "biphasic pattern"). Clinically, the platelet count will drop until the patient's temperature is normal. Cases of DHF also show higher fever, variable hemorrhagic phenomena, thrombocytopenia, and hemoconcentration. A small proportion of cases lead to dengue shock syndrome (DSS) which has a high mortality rate.
Diagnosis of dengue fever:
The diagnosis of dengue is usually made clinically. The classic picture is high fever with no localising source of infection, a petechial rash with thrombocytopenia and relative leukopenia - low platelet and white blood cell count. Care has to be taken as diagnosis of DHF can mask end stage liver disease and vice versa.
- Fever, bladder problem, constant headaches, eye pain, severe dizziness and loss of appetite.
- Hemorrhagic tendency (positive tourniquet test, spontaneous bruising, bleeding from mucosa, gingiva, injection sites, etc.; vomiting blood, or bloody diarrhea)
- Thrombocytopenia (<100,000 platelets per mm³ or estimated as less than 3 platelets per high power field)
- Evidence of plasma leakage (hematocrit more than 20% higher than expected, or drop in hematocrit of 20% or more from baseline following IV fluid, pleural effusion, ascites, hypoproteinemia)
- Encephalitic occurrences.
Dengue shock syndrome is defined as dengue hemorrhagic fever plus:
- Weak rapid pulse,
- Narrow pulse pressure (less than 20 mm Hg)
- Cold, clammy skin and restlessness.
Dependable, immediate diagnosis of dengue can be performed in rural areas by the use of Rapid Diagnostic Test kits, which also differentiate between primary and secondary dengue infections.[123] Serology and polymerase chain reaction (PCR) studies are available to confirm the diagnosis of dengue if clinically indicated. Dengue can be a life threatening fever.
Cause of Dengue fever: Dengue fever is caused by Dengue virus (DENV), a mosquito-borne flavivirus. DENV is an ssRNA positive-strand virus of the family Flaviviridae; genus Flavivirus. There are four serotypes of DENV. The virus has a genome of about 11000 bases that codes for three structural proteins, C, prM, E; seven nonstructural proteins, NS1, NS2a, NS2b, NS3, NS4a, NS4b, NS5; and short non-coding regions on both the 5' and 3' ends.[124]
The potential factors causing hemorrhagic fever are varied. The most suspected factors are human's cross-serotypic immune response and membrane fusion process.
Meningococcal disease
[edit]- Meningococcal disease, including meningococcal meningitis.[125]
Meningococcal disease describes infections caused by the bacterium Neisseria meningitidis (also termed meningococcus). It carries a high mortality rate if untreated. Whilst best known as a cause of meningitis, wide spread blood infection (sepsis) is more damaging and dangerous. Meningitis and Meningococcemia are major causes of illness, death and disability in both developed and under developed countries worldwide.
The disease's host/pathogen interaction is not fully understood. The pathogen originates harmlessly in a large number of the general population, but thereafter can invade the blood stream and the brain, causing serious illness. Over the past few years, experts have made an intensive effort to understand specific aspects of meningococcal biology and host interactions, however the development of improved treatments and effective vaccines will depend on novel efforts by workers in many different fields.[126]
The incidence of endemic meningococcal disease during the last 13 years ranges from 1 to 5 per 100,000 in developed countries, and from 10 to 25 per 100,000 in developing countries. During epidemics the incidence of meningococcal disease approaches 1000 per 1,000,000. There are approximately 2,600 cases of bacterial meningitis per year in the United States, and on average 333,000 cases in developing countries. The case fatality rate ranges between 10 and 20 per cent.[127].
While Meningococcal disease is not as contagious as the common cold (which is spread through casual contact), it can be transmitted through saliva and occasionally through close, prolonged general contact with an infected person.
Pathogenesis of meningococcal disease: Meningococcal disease causes life-threatening meningitis and sepsis conditions. In the case of meningitis, bacteria attack the lining between the brain and skull called the meninges. Infected fluid from the meninges then passes into the spinal cord, causing symptoms including stiff neck, fever and rashes. The meninges (and sometimes the brain itself) begin to swell, which affects the central nervous system.
Even with antibiotics, approximately 1 in 10 victims of meningococcal meningitis will die; However, about as many survivors of the disease lose a limb or their hearing, or suffer permanent brain damage.[128] The sepsis type of infection is much more deadly, and results in a severe blood poisoning called meningococcal sepsis that affects the entire body. In this case, bacterial toxins rupture blood vessels and can rapidly shut down vital organs. Within hours, patient's health can change from seemingly good to mortally ill.[129].
The N. meningitidis bacterium is surrounded by a slimy outer coat that contains disease-causing endotoxin. While many bacteria produce endotoxin, the levels produced by meningococcal bacteria are 100 to 1,000 times greater (and accordingly more lethal) than normal. As the bacteria multiply and move through the bloodstream, it sheds concentrated amounts of toxin. The endotoxin directly affects the heart, reducing its ability to circulate blood, and also causes pressure on blood vessels throughout the body. As some blood vessels start to hemorrhage, major organs like the lungs and kidneys are damaged.
Patients suffering from meningococcal disease are treated with a large dose of antibiotic. The systemic antibiotic flowing through the bloodstream rapidly kills the bacteria but, as the bacteria are killed, even more toxin is released. It takes up to several days for the toxin to be neutralized from the body by using continuous liquid treatment and antibiotic therapy[130]. Signs and symptoms of meningococcal disease Meningitis: The patient with meningigococcal meningitis typically presents with high fever, meningism (stiff neck), Kernig's sign, severe headache, vomiting, purpura, photophobia, and sometimes chills, altered mental status, or seizures. Diarrhea or respiratory symptoms are less common. Petechiae is often also present, but does not always occur, so its absence should not be used against the diagnosis of meningococcal disease. Anyone with symptoms of meningococcal meningitis should receive intravenus antibiotics pending results of lumbar puncture, as delay in treatment worsens the prognosis. Meningococcemia: Symptoms of meningococcemia are, at least initially, similar to those of influenza. Typically, the first symptoms include fever, nausea, myalgia, headache, arthralgia, chills, diarrhea, and malaise. Later symptoms include septic shock, purpura, hypotension, cyanosis, petechiae, seizures, anxiety, and multiple organ dysfunction syndrome. Acute respiratory distress syndrome and altered mental status may also occur. Meningococcal sepsis has a higher mortality rate then meningococcal meningitis, but the risk of neurologic sequalae is much lower.[citation needed]
Types of infection: Meningococcemia: Meningococcemia, like many gram-negative blood infections, can cause disseminated intravascular coagulation (DIC), a condition where blood starts to clot throughout the body, sometimes causing ischemic tissue damage. DIC also causes bleeding, when the clotting factors are used up, causing the characteristic purpuric rash.
Meningitis: Meningococcal meningitis is a consequence of bacteria entering the cerebrospinal fluid (CSF) and irritating the meninges - the membranes that line the brain and spinal cord. Sub-Saharan Africa, Americas, Western Europe, UK and Ireland face multifarious challenges, 200 years after the discovery of bacterial meningitis[131].
Other types of infection: As with any gram negative bacterium, N. meningitidis can infect a variety of sites.
Meningococcal pneumonia can appear during influenza pandemics and in military camps. This is a multilobar, rapidly evolving pneumonia, sometimes associated with septic shock. With prompt treatment with penicillin or chloramphenicol, the prognosis is excellent. Pericarditis can appear, either as a septic pericarditis with grave prognosis or as a rective pericarditis in the wake of meningitis or septicaemia. Myocarditis can be a complication of meningococcemia and can be contributive to shock seen in this form of disease. Pharyngitis and conjunctivitis can also appear and can constitute the portal of entry for the bacterium. Septic arthritis due to N. meningitidis can be seen, usually accompanying disseminated infection. Other forms of disease can rarely be seen, like osteomyelitis, endophthalmitis and urethritis.
Hantavirus
[edit]Hantaviruses belong to the Bunyaviridae family of viruses. The Bunyaviridae family is divided into 5 genera: Orthobunyavirus, Nairovirus, Phlebovirus, Tospovirus, and Hantavirus. Like all members of this family, hantaviruses have genomes comprising three negative-sense, single-stranded RNA segments, and so are classified as negative sense RNA viruses. Viruses in the genus Hantavirus are unique in that they are transmitted by aerosolized rodent excreta or rodent bites, whereas all other genera in the Bunyaviridae family are arthropod-borne viruses.
The name hantavirus is derived from the Hantan River, where the Hantaan virus (the etiologic agent of Korean hemorrhagic fever) was first isolated by Dr. Ho-Wang Lee and colleagues. The disease associated with Hantaan virus is called hemorrhagic fever with renal syndrome (HFRS), a term that is accepted by the World Health Organization. It was formerly called Korean hemorrhagic fever (a term that is no longer in use).
History of hantavirus infection: The hantaviruses constitute a relatively newly discovered genus of viruses; the disease entity HFRS was first recognized by Korean Dr.Lee Ho Wang who worked for Western medicine during the Korean War in the early 1950s.
In 1993, a newly-recognized species of hantavirus was found to be behind the Hantavirus cardiopulmonary syndrome (HCPS, also called HPS) caused by the Sin Nombre virus (in Spanish, "Virus sin nombre", for "nameless virus") in New Mexico and other Four Corners states. It was first identified by Terry Yates, a professor at the University of New Mexico.[133] In addition to Hantaan virus and Sin Nombre virus, several other hantaviruses have been implicated as etiologic agents for either HFRS or HCPS. Virology and Genome: Like other members of the bunyavirus family, hantaviruses are enveloped viruses with a genome that consists of three single-stranded, negative sense RNA segments designated S (small), M (medium), and L (large). The S RNA encodes the nucleocapsid (N) protein. The M RNA encodes a polyprotein that is cotranslationally cleaved to yield the envelope glycoproteins G1 and G2. The L RNA encodes the L protein, which functions as the viral transcriptase/replicase. Within virions, the genomic RNAs of hantaviruses are thought to complex with the N protein to form helical nucleocapsids, the RNA component of which circularizes due to sequence complementarity between the 5' and 3' terminal sequences of genomic segments.
Entry into host cells is thought to occur by attachment of virions to cellular receptors and subsequent endocytosis. Nucleocapsids are introduced into the cytoplasm by pH-dependent fusion of the virion with the endosomal membrane. Subsequent to release of the nucleocapsids into cytoplasm, the complexes are targeted to the ER-Golgi Intermediate compartments (ERGIC) through microtubular associated movement resulting in the formation of viral factories at ERGIC. These factories then facilitate transcription and subsequent translation of the viral proteins. Transcription of viral genes must be initiated by association of the L protein with the three nucleocapsid species. In addition to transcriptase and replicase functions, the viral L protein is also thought to have an endonuclease activity that cleaves cellular messenger RNAs (mRNAs) for the production of capped primers used to initiate transcription of viral mRNAs. As a result of this "cap snatching," the mRNAs of hantaviruses are capped and contain nontemplated 5' terminal extensions. The G1 (aka Gn) and G2 (Gc) glycoproteins form hetero-oligomers and are then transported from the endoplasmic reticulum to the Golgi complex, where glycosylation is completed. The L protein produces nascent genomes by replication via a positive-sense RNA intermediate. Hantavirus virions are believed to assemble by association of nucleocapsids with glycoproteins embedded in the membranes of the Golgi, followed by budding into the Golgi cisternae. Nascent virions are then transported in secretory vesicles to the plasma membrane and released by exocytosis.
Pathogenesis of hantavirus infection: The pathogenesis of Hantavirus infections is unclear as there is a lack of animal models (rats and mice do not seem to acquire severe disease). While the primary replication site is not clear, in both HFRS and HPS, the main effect is in the blood vessels. There is increased vascular permeability and decreased blood pressure due to endothelial dysfunction. In HFRS, the most dramatic damage is seen in the kidneys, whereas in HPS, the lungs, spleen, and gall bladder are most affected.
[135] WNV has three different effects on humans. The first is an asymptomatic infection; the second is a mild febrile syndrome termed West Nile Fever;[1] the third is a neuroinvasive disease termed West Nile meningitis or encephalitis.[2] In many infected individuals the ratio between the three states is roughly 110:30:1.[3] The second, febrile stage has an incubation period of 2 to 8 days followed by fever, headache, chills, diaphoresis (excessive sweating), weakness, lymphadenopathy (swollen lymph nodes), drowsiness, pain in the joints and symptoms like those of influenza or the flu. Occasionally there is a short-lived truncal rash and some patients experience gastrointestinal symptoms including nausea, vomiting, loss of appetite, or diarrhea. All symptoms are resolved within 7 to 10 days, although fatigue can last for some weeks and lymphadenopathy can take up to two months to resolve. The more dangerous encephalitis is characterized by similar early symptoms but also a decreased level of consciousness, sometimes approaching near-coma. Deep tendon reflexes are hyperactive at first, later diminished. There are also extrapyramidal disorders. Recovery is marked by a long convalescence with fatigue. An atypical case of West Nile encephalitis presenting as jaw pain has been described. More recent outbreaks have resulted in a deeper study of the disease and other, rarer, outcomes have been identified. The spinal cord may be infected, marked by anterior myelitis with or without encephalitis.[4] WNV-associated Guillain-Barré syndrome has been identified[5] and other rare effects include multifocal chorioretinitis (which has 100% specificity for identifying WNV infection in patients with possible WNV encephalitis),[6] hepatitis, myocarditis, nephritis, pancreatitis, and splenomegaly.[7][8][9]
Acute HIV infection
[edit]Acute HIV infection or primary HIV infection (also known as "Acute seroconversion syndrome"[136]: 416 ) is the first stage of HIV infection. It occurs before the latency stage and the potential AIDS succeeding the latency stage.
Symptoms of Acute HIV infection: During this period (usually 2–4 weeks post-exposure) most individuals (80 to 90%) develop influenza or mononucleosis-like symptoms, most commonly fever, lymphadenopathy, pharyngitis, generalized rash of maculopapular[137] type, myalgia, malaise, mouth and esophagal sores, and may also include, but less commonly, headache, nausea and vomiting, enlarged liver/spleen, weight loss, thrush, and neurological symptoms. Infected individuals may experience all, some, or none of these symptoms. The acute illness can last between a few days and 10 weeks, though usually less than 14 days.[138] In some very rare cases, about 10 patients from 1989 to date[139], a bilateral facial palsy has been associated with acute HIV-1 infection.
Because of the nonspecific nature of these symptoms, they are often not recognized as signs of HIV infection. Even if patients go to their doctors or a hospital, they will often be misdiagnosed as having one of the more common infectious diseases with the same symptoms. Consequently, these primary symptoms are not used to diagnose HIV infection as they do not develop in all cases and because many are caused by other more common diseases. However, recognizing the syndrome can be important because the patient is much more infectious during this period. [140]
These nonspecific symptoms can also be symptoms of other infections; consequently, having these symptoms does not reliably indicate the presence of HIV.
Lyme disease, or lyme borreliosis,[141] is an emerging infectious disease caused by at least three species of bacteria belonging to the genus Borrelia.[122] Borrelia burgdorferi sensu lato[142] is the main cause of Lyme disease in the United States, whereas Borrelia afzelii and Borrelia garinii cause most European cases. The disease is named after the village of Lyme, Connecticut, USA, where a number of cases were identified in 1975. Although Allen Steere realized in 1978 that Lyme disease was a tick-borne disease, the cause of the disease remained a mystery until 1982, when B. burgdorferi was identified by Willy Burgdorfer.
Lyme disease is the most common tick-borne disease in the Northern Hemisphere. Borrelia is transmitted to humans by the bite of infected ticks belonging to a few species of the genus Ixodes ("hard ticks").[4] Early symptoms may include fever, headache, fatigue, depression, and a characteristic circular skin rash called erythema migrans. Left untreated, later symptoms may involve the joints, heart, and central nervous system. In most cases, the infection and its symptoms are eliminated by antibiotics, especially if the illness is treated early.[citation needed] Late, delayed, or inadequate treatment can lead to the more serious symptoms, which can be disabling and difficult to treat.[143] Occasionally, symptoms such as arthritis persist after the infection has been eliminated by antibiotics, prompting suggestions that Borrelia causes autoimmunity.[144] Lyme disease, is an increasingly common tick-borne spirochetal infection.[145]
[146]Chickenpox or chicken pox is a highly contagious illness caused by primary infection with varicella zoster virus (VZV).[147] It usually starts with vesicular skin rash mainly on the body and head rather than at the periphery and become itchy raw pockmarks which mostly heal without scarring.
Chicken pox is spread easily through coughs or sneezes of ill individuals, or through direct contact with secretions from the rash. Following primary infection there is usually lifelong protective immunity from further episodes of chickenpox.
Chickenpox is rarely fatal, although it is generally more severe in adult males than in adult females or children. Pregnant women and those with a suppressed immune system are at highest risk of serious complications. Chicken pox is now believed to be the cause of one third of stroke cases in children.[148] The most common late complication of chicken pox is shingles, caused by reactivation of the varicella zoster virus decades after the initial episode of chickenpox.
Measles is an infection of the respiratory system caused by a virus, specifically a paramyxovirus of the genus Morbillivirus. Morbilliviruses, like other paramyxoviruses, are enveloped, single-stranded, negative-sense RNA viruses. Symptoms include fever, cough, runny nose, red eyes and a generalized, maculopapular, erythematous rash.
Measles is spread through respiration (contact with fluids from an infected person's nose and mouth, either directly or through aerosol transmission), and is highly contagious—90% of people without immunity sharing a house with an infected person will catch it. The infection has an average incubation period of 14 days (range 6–19 days) and infectivity lasts from 2–4 days prior, until 2–5 days following the onset of the rash (i.e. 4–9 days infectivity in total).[150]
An alternative name for measles in English-speaking countries is rubeola, which is sometimes confused with rubella (German measles); the diseases are unrelated.[151][152] In some other European languages, rubella and rubeola are synonyms, and rubeola is not an alternative name for measles.[153]
[154] Mumps and epidemic parotitis is a viral disease of the human species, caused by the mumps virus. Prior to the development of vaccination and the introduction of a vaccine, it was a common childhood disease worldwide, and is still a significant threat to health in the third world.[1] Painful swelling of the salivary glands (classically the parotid gland) is the most typical presentation.[2] Painful testicular swelling (orchitis) and rash may also occur. The symptoms are generally not severe in children. In teenage males and men, complications such as infertility or subfertility are more common, although still rare in absolute terms.[3][4][5] The disease is generally self-limited, running its course before receding, with no specific treatment apart from controlling the symptoms with painkillers.
May sometimes cause fever, particulary in adults.[155] Rubella, commonly known as German measles, is a disease caused by the rubella virus. The name "rubella" is derived from the Latin, meaning little red. Rubella is also known as German measles because the disease was first described by German physicians in the mid-eighteenth century. This disease is often mild and attacks often pass unnoticed. The disease can last one to three days. Children recover more quickly than adults. Infection of the mother by Rubella virus during pregnancy can be serious; if the mother is infected within the first 20 weeks of pregnancy, the child may be born with congenital rubella syndrome (CRS), which entails a range of serious incurable illnesses. Spontaneous abortion occurs in up to 20% of cases.[1] Rubella is a common childhood infection usually with minimal systemic upset although transient arthropathy may occur in adults. Serious complications are very rare. Apart from the effects of transplacental infection on the developing fetus, rubella is a relatively trivial infection. Acquired (i.e. not congenital) rubella is transmitted via airborne droplet emission from the upper respiratory tract of active cases. The virus may also be present in the urine, feces and on the skin. There is no carrier state: the reservoir exists entirely in active human cases. The disease has an incubation period of 2 to 3 weeks.[2] In most people the virus is rapidly eliminated. However, it may persist for some months post partum in infants surviving the CRS. These children are a significant source of infection to other infants and, more importantly, to pregnant female contacts. The name rubella is sometimes confused with rubeola, an alternative name for measles in English-speaking countries; the diseases are unrelated.[3][4] In some other European languages, rubella and rubeola are synonyms, and rubeola is not an alternative name for measles.[5]
- Common cold [156]
- Eastern equine encephalitis virus[157]
- Viral gastroenteritis[158]
- Rocky mountain spotted fever[159]
- Appendicitis[160]
- Schistosomiasis[161]
- Meningitis[162]
- Babesiosis[citation needed]
- Tularemia[163]
- Typhus[164]
- Relapsing fever[165]
Typhoid fever, also known as Salmonella Typhi or commonly just typhoid,[167] is a common worldwide illness, transmitted by the ingestion of food or water contaminated with the feces of an infected person.[4] The bacteria then perforate through the intestinal wall and are phagocytosed by macrophages. The organism is a Gram-negative short bacillus that is motile due to its peritrichous flagella. The bacterium grows best at 37 °C (99 °F)* – human body temperature.
This fever received various names, such as gastric fever, the bends, abdominal typhus, infantile remittent fever, slow fever, nervous fever, pythogenic fever, etc. The name of " typhoid " was given by Louis in 1829, as a derivative from typhus.
The impact of this disease falls sharply with the application of modern sanitation techniques.
Typhoid fever is characterized by a slowly progressive fever as high as 40 °C (104 °F), profuse sweating, gastroenteritis, and nonbloody diarrhea. Less commonly a rash of flat, rose-colored spots may appear.[168]
Classically, the course of untreated typhoid fever is divided into four individual stages, each lasting approximately one week. In the first week, there is a slowly rising temperature with relative bradycardia, malaise, headache and cough. A bloody nose (epistaxis) is seen in a quarter of cases and abdominal pain is also possible. There is leukopenia, a decrease in the number of circulating white blood cells, with eosinopenia and relative lymphocytosis, a positive diazo reaction and blood cultures are positive for Salmonella typhi or paratyphi. The classic Widal test is negative in the first week.
In the second week of the infection, the patient lies prostrated with high fever in plateau around 40 °C (104 °F) and bradycardia (Sphygmo-thermic dissociation), classically with a dicrotic pulse wave. Delirium is frequent, frequently calm, but sometimes agitated. This delirium gives to typhoid the nickname of "nervous fever". Rose spots appear on the lower chest and abdomen in around 1/3 patients. There are rhonchi in lung bases. The abdomen is distended and painful in the right lower quadrant where borborygmi can be heard. Diarrhea can occur in this stage: six to eight stools in a day, green with a characteristic smell, comparable to pea-soup. However, constipation is also frequent. The spleen and liver are enlarged (hepatosplenomegaly) and tender and there is elevation of liver transaminases. The Widal reaction is strongly positive with antiO and antiH antibodies. Blood cultures are sometimes still positive at this stage. (The major symptom of this fever is the fever usually rises in the afternoon up to the first and second week.)
In the third week of typhoid fever a number of complications can occur:
- Intestinal hemorrhage due to bleeding in congested Peyer's patches; this can be very serious but is usually non-fatal.
Fever typically coincides with severe abdominal pain[169]
Peritonitis is an inflammation of the peritoneum, the serous membrane which lines part of the abdominal cavity and viscera. Peritonitis may be localized or generalized, and may result from infection (often due to rupture of a hollow organ as may occur in abdominal trauma or appendicitis) or from a non-infectious process.
The main manifestations of peritonitis are acute abdominal pain, abdominal tenderness, and abdominal guarding, which are exacerbated by moving the peritoneum, e.g. coughing (forced cough may be used as a test), flexing one's hips, or eliciting the Blumberg sign (a.k.a. rebound tenderness, meaning that pressing a hand on the abdomen elicits less pain than releasing the hand abruptly, which will aggravate the pain, as the peritoneum snaps back into place). The presence of these signs in a patient is sometimes referred to as peritonism.[170] The localization of these manifestations depends on whether peritonitis is localized (e.g. appendicitis or diverticulitis before perforation), or generalized to the whole abdomen. In either case pain typically starts as a generalized abdominal pain (with involvement of poorly localizing innervation of the visceral peritoneal layer), and may become localized later (with the involvement of the somatically innervated parietal peritoneal layer). Peritonitis is an example of an acute abdomen.
- Diffuse abdominal rigidity ("washboard abdomen") is often present, especially in generalized peritonitis
- Fever
- Sinus tachycardia
- Development of ileus paralyticus (i.e. intestinal paralysis), which also causes nausea and vomiting
Complications of peritonitis
- Sequestration of fluid and electrolytes, as revealed by decreased central venous pressure, may cause electrolyte disturbances, as well as significant hypovolemia, possibly leading to shock and acute renal failure.
- A peritoneal abscess may form (e.g. above or below the liver, or in the lesser sac
- Sepsis may develop, so blood cultures should be obtained.
- The fluid may push on the diaphragm causing splinting and subsequent breathing difficulties.
[171] Necrotizing fasciitis (NF), commonly known as flesh-eating disease or flesh-eating bacteria, is a rare infection of the deeper layers of skin and subcutaneous tissues, easily spreading across the fascial plane within the subcutaneous tissue.
Type I describes a polymicrobial infection, whereas Type II describes a monomicrobial infection. Many types of bacteria can cause necrotizing fasciitis (e.g., Group A streptococcus (Streptococcus pyogenes), Staphylococcus aureus, Vibrio vulnificus, Clostridium perfringens, Bacteroides fragilis). Such infections are more likely to occur in people with compromised immune systems.[172]
Historically, Group A streptococcus made up most cases of Type II infections. However, since at least 2001, another serious form of monomicrobial necrotizing fasciitis has been observed with increasing frequency.[173] In these cases, the bacterium causing it is methicillin-resistant Staphylococcus aureus (MRSA), a strain of S. aureus that is resistant to methicillin, the antibiotic used in the laboratory that determines the bacterium's sensitivity to flucloxacillin or nafcillin that would be used for treatment clinically.
Symptoms of necrotizing fasciitis: The infection begins locally, at a site of trauma, which may be severe (such as the result of surgery), minor, or even non-apparent. Patients usually complain of intense pain that may seem in excess given the external appearance of the skin. With progression of the disease, tissue becomes swollen, often within hours. Diarrhea and vomiting are also common symptoms.
In the early stages, signs of inflammation may not be apparent if the bacteria are deep within the tissue. If they are not deep, signs of inflammation, such as redness and swollen or hot skin, show very quickly. Skin color may progress to violet, and blisters may form, with subsequent necrosis (death) of the subcutaneous tissues.
Patients with necrotizing fasciitis typically have a fever and appear very ill. Mortality rates have been noted as high as 73 percent if left untreated.[174] Without surgery and medical assistance, such as antibiotics, the infection will rapidly progress and will eventually lead to death.[175]
Cellulitis is a diffuse inflammation[176] of connective tissue with severe inflammation of dermal and subcutaneous layers of the skin. Cellulitis can be caused by normal skin flora or by exogenous bacteria, and often occurs where the skin has previously been broken: cracks in the skin, cuts, blisters, burns, insect bites, surgical wounds, or sites of intravenous catheter insertion. Skin on the face or lower legs is most commonly affected by this infection, though cellulitis can occur on any part of the body. The mainstay of therapy remains treatment with appropriate antibiotics.
Erysipelas is the term used for a more superficial infection of the dermis and upper subcutaneous layer that presents clinically with a well defined edge. Erysipelas and cellulitis often coexist, so it is often difficult to make a distinction between the two.
Cellulitis is unrelated (except etymologically) to cellulite, a cosmetic condition featuring dimpling of the skin.
Cellulitis is caused by a type of bacteria entering the skin, usually by way of a cut, abrasion, or break in the skin. This break does not need to be visible. Group A Streptococcus and Staphylococcus are the most common of these bacteria, which are part of the normal flora of the skin but cause no actual infection while on the skin's outer surface. Predisposing conditions for cellulitis include insect bites, blistering, animal bite, tattoos, pruritic skin rash, recent surgery, athlete's foot, dry skin, eczema, injecting drugs (especially subcutaneous or intramuscular injection or where an attempted IV injection "misses" or blows the vein), pregnancy, diabetes and obesity, which can affect circulation, as well as burns and boils, though there is debate as to whether minor foot lesions contribute.
There is a very rare dermatological condition called Hidradenitis Suppurativa (HS). HS is a non-contagious skin disease that includes numerous and chronic manifestaions of symptoms, including abscesses, several types of cysts (epidermoid, sebaceous, pilonidal), just to name a few. These infections of the skin usually occurs in clusters, leading to multilocalized infections that are extremely sensitive and often cause debilitating pain. Persistent and reoccuring lesions can cause scarring and may cause the formation of sinus tracts depending on the extent of the wounds. These lesions can also cause the formation of tunnels connecting the abscesses or infections underneath the skin. Most patients with HS require multiple surgeries in order to be able to live somewhat comfortably. Wound dehiscence is a premature bursting open of the wounds along surgical sutures, which only further complicates the resistant healing process. Wound dehiscence can be considered a surgical complication, although it occurs very frequently in patients with Hidradenitis Suppurativa who have undergone surgery specifically for their skin wounds. Occurrences of bacterial infections and cellulitis (deep tissue inflammation) often occur at these sites. Hidradenitis Suppurativa pain and depression can be extremely difficult to manage [177]. HS has been linked with other auto-immune conditions[178], androgen dysfunction, plugged or dysfunctional apocrine (sweat) glands[179] and/or hair follicles[180] (creates inflammation, pain, and swollen lesions; and there may be an autoimmune component implied)), bacterial infections (which are infections that are secondary infections and therefore not contagious), Crohn's Disease, Rheumatoid Arthiritis, Squamous Cell Carcinoma, Hashimoto's Thyroiditis, obesity, anemia, amyloidosis, arthropathy. Stage III Hidradenitis Suppurative complications are extremely painful and can lead to death in some cases.
The photos shown here of Cellulitis are more severe cases than what can be spotted normally at earlier stages. Usually the itch and/or rash appears a little after it vanishes leaving only a small mark which is commonly ignored.
The appearance of the skin will help a doctor make a diagnosis. The doctor may also suggest blood tests, a wound culture or other tests to help rule out a blood clot deep in the veins of the legs. Cellulitis in the lower leg is characterized by signs and symptoms that may be similar to those of a clot occurring deep in the veins, such as warmth, pain and swelling (inflammation).
This reddened skin or rash may signal a deeper, more serious infection of the inner layers of skin. Once below the skin, the bacteria can spread rapidly, entering the lymph nodes and the bloodstream and spreading throughout the body.
In rare cases, the infection can spread to the deep layer of tissue called the fascial lining. Necrotizing fasciitis, also called by the media "flesh-eating bacteria," is an example of a deep-layer infection. It represents an extreme medical emergency.
Risk factors for cellulitis: The elderly and those with immunodeficiency (a weakened immune system) are especially vulnerable to contracting cellulitis. Diabetics are more susceptible to cellulitis than the general population because of impairment of the immune system; they are especially prone to cellulitis in the feet because the disease causes impairment of blood circulation in the legs leading to diabetic foot/foot ulcers. Poor control of blood glucose levels allows bacteria to grow more rapidly in the affected tissue and facilitates rapid progression if the infection enters the bloodstream. Neural degeneration in diabetes means these ulcers may not be painful and thus often become infected.
Immunosuppressive drugs, and other illnesses or infections that weaken the immune system are also factors that make infection more likely. Chickenpox and shingles often result in blisters that break open, providing a gap in the skin through which bacteria can enter. Lymphedema, which causes swelling on the arms and/or legs, can also put an individual at risk.
Diseases that affect blood circulation in the legs and feet, such as chronic venous insufficiency and varicose veins, are also risk factors for cellulitis.
Cellulitis is also extremely prevalent among dense populations sharing hygiene facilities and common living quarters, such as military installations, college dormitories, and homeless shelters.
Diagnosing cellulitis: Cellulitis is most often a clinical diagnosis, and local cultures do not always identify the causative organism. Blood cultures usually are positive only if the patient develops generalized sepsis. Conditions that may resemble cellulitis include deep vein thrombosis, which can be diagnosed with a compression leg ultrasound, and stasis dermatitis, which is inflammation of the skin from poor blood flow.
Yellow fever
[edit][181] Yellow fever is an acute viral hemorrhagic disease.[4] The virus is a 40 to 50 nm enveloped RNA virus with positive sense of the Flaviviridae family.
The yellow fever virus is transmitted by the bite of female mosquitos (the yellow fever mosquito, Aedes aegypti, and other species) and is found in tropical and subtropical areas in South America and Africa, and Asia.[182] The only known hosts of the virus are primates and several species of mosquito. The origin of the disease is most likely to be Africa, from where it was introduced to South America through the slave trade in the 16th century. Since the 17th century, several major epidemics of the disease have been recorded in the Americas, Africa and Europe. In the 19th century, yellow fever was deemed one of the most dangerous infectious diseases.[183]
Clinically, yellow fever presents in most cases with fever, nausea, and pain and it generally subsides after several days. In some patients, a toxic phase follows, in which liver damage with jaundice (giving the name of the disease) can occur and lead to death. Because of the increased bleeding tendency (bleeding diathesis), yellow fever belongs to the group of hemorrhagic fevers. The WHO estimates that yellow fever causes 200,000 illnesses and 30,000 deaths every year in unvaccinated populations;[184] around 90% of the infections occur in Africa.[185]
A safe and effective vaccine against yellow fever has existed since the middle of the 20th century and some countries require vaccinations for travelers.[122] Since no therapy is known, vaccination programs are, along with measures to reduce the population of the transmitting mosquito, of great importance in affected areas. Since the 1980s, the number of cases of yellow fever has been increasing, making it a reemerging disease.[186]
Signs and symptoms of Yellow fever: Yellow fever begins suddenly after an incubation period of three to six days. Most cases only cause a mild infection with fever, headache, chills, back pain, loss of appetite, nausea and vomiting.[187] In these cases the infection lasts only three to four days. 15% of cases enter a second, toxic phase of the disease with recurring fever, this time accompanied by jaundice due to liver damage, as well as abdominal pain. Bleeding in the mouth, the eyes and in the gastrointestinal tract can cause vomitus containing blood (giving the name "vómito negro").[188] The toxic phase is fatal in approximately 20% of cases.[189]
Smallpox
[edit][190] Smallpox is an infectious disease unique to humans, caused by either of two virus variants, Variola major and Variola minor.[122] The disease is also known by the Latin names Variola or Variola vera, which is a derivative of the Latin varius, meaning spotted, or varus, meaning "pimple". The term "smallpox" was first used in Europe in the 15th century to distinguish variola from the "great pox" (syphilis).[191]
Smallpox localizes in small blood vessels of the skin and in the mouth and throat. In the skin, this results in a characteristic maculopapular rash, and later, raised fluid-filled blisters. V. major produces a more serious disease and has an overall mortality rate of 30–35%. V. minor causes a milder form of disease (also known as alastrim, cottonpox, milkpox, whitepox, and Cuban itch) which kills about 1% of its victims.[192][193] Long-term complications of V. major infection include characteristic scars, commonly on the face, which occur in 65–85% of survivors.[194] Blindness resulting from corneal ulceration and scarring, and limb deformities due to arthritis and osteomyelitis are less common complications, seen in about 2–5% of cases.
Smallpox is believed to have emerged in human populations about 10,000 BC.[191] The disease killed an estimated 400,000 Europeans per year during the closing years of the 18th century (including five monarchs),[195] and was responsible for a third of all blindness.[192][196] Of all those infected, 20–60%—and over 80% of infected children—died from the disease.[197]
During the 20th century, it is estimated that smallpox was responsible for 300–500 million deaths.[198][199][200] In the early 1950s an estimated 50 million cases of smallpox occurred in the world each year.[201] As recently as 1967, the World Health Organization (WHO) estimated that 15 million people contracted the disease and that two million died in that year.[201] After successful vaccination campaigns throughout the 19th and 20th centuries, the WHO certified the eradication of smallpox in December 1979.[201] To this day, smallpox is the only human infectious disease to have been eradicated.[202]
Cause: Smallpox is caused by infection with variola virus, which belongs to the genus Orthopoxvirus, the family Poxviridae, and subfamily chordopoxvirinae. Variola is a large brick-shaped virus measuring approximately 302 to 350 nanometers by 244 to 270 nm,[203] with a single linear double stranded DNA genome 186 kilobase pairs (kbp) in size and containing a hairpin loop at each end.[204][205] The two classic varieties of smallpox are variola major and variola minor. The closest viral relative is molluscum contagiosum, which, like smallpox, infects only humans. However, unlike variola species, molluscum infection is benign.
Four orthopoxviruses cause infection in humans: variola, vaccinia, cowpox, and monkeypox. Variola virus infects only humans in nature, although primates and other animals have been infected in a laboratory setting. Vaccinia, cowpox, and monkeypox viruses can infect both humans and other animals in nature.[206]
The lifecycle of poxviruses is complicated by having multiple infectious forms, with differing mechanisms of cell entry. Poxviruses are unique among DNA viruses in that they replicate in the cytoplasm of the cell rather than in the nucleus. In order to replicate, poxviruses produce a variety of specialized proteins not produced by other DNA viruses, the most important of which is a viral-associated DNA-dependent RNA polymerase. Both enveloped and unenveloped virions are infectious. The viral envelope is made of modified Golgi membranes containing viral-specific polypeptides, including hemagglutinin.[204] Infection with either variola major or variola minor confers immunity against the other.[193]
Transmission of Smallpox Transmission of smallpox occurs through inhalation of airborne variola virus, usually droplets expressed from the oral, nasal, or pharyngeal mucosa of an infected person. It is transmitted from one person to another primarily through prolonged face-to-face contact with an infected person, usually within a distance of 6 feet, but can also be spread through direct contact with infected bodily fluids or contaminated objects (fomites) such as bedding or clothing. Rarely, smallpox has been spread by virus carried in the air in enclosed settings such as buildings, buses, and trains.[207] The virus can cross the placenta, but the incidence of congenital smallpox is relatively low.[193] Smallpox is not notably infectious in the prodromal period and viral shedding is usually delayed until the appearance of the rash, which is often accompanied by lesions in the mouth and pharynx. The virus can be transmitted throughout the course of the illness, but is most frequent during the first week of the rash, when most of the skin lesions are intact.[206] Infectivity wanes in 7 to 10 days when scabs form over the lesions, but the infected person is contagious until the last smallpox scab falls off.[208]
Smallpox is highly contagious, but generally spreads more slowly and less widely than some other viral diseases, perhaps because transmission requires close contact and occurs after the onset of the rash. The overall rate of infection is also affected by the short duration of the infectious stage. In temperate areas, the number of smallpox infections were highest during the winter and spring. In tropical areas, seasonal variation was less evident and the disease was present throughout the year.[206] Age distribution of smallpox infections depends on acquired immunity. Vaccination immunity declines over time and is probably lost in all but the most recently vaccinated populations.[193] Smallpox is not known to be transmitted by insects or animals and there is no asymptomatic carrier state.[206]
Signs and symptoms of smallpox: There are two clinical forms of smallpox. Variola major is the severe and most common form of smallpox, with a more extensive rash and higher fever. There are four types of variola major smallpox based on the Rao classification:[209] ordinary, modified, flat, and hemorrhagic. Historically, variola major has an overall fatality rate of about 30%; however, flat and hemorrhagic smallpox are usually fatal.[210] In addition, a form called variola sine eruptione (smallpox without rash) is seen generally in vaccinated persons. This form is marked by a fever that occurs after the usual incubation period and can be confirmed only by antibody studies or, rarely, by virus isolation.[206]
Variola minor is a less common presentation of smallpox, and a much less severe disease, with historical death rates of 1% or less.[207] Subclinical (asymptomatic) infections with variola virus have also been noted, but are not believed to be common.[206] The incubation period between contraction and the first obvious symptoms of the disease is around 12 days. Once inhaled, variola virus invades the oropharyngeal (mouth and throat) or the respiratory mucosa, migrates to regional lymph nodes, and begins to multiply. In the initial growth phase the virus seems to move from cell to cell, but around the 12th day, lysis of many infected cells occurs and the virus is found in the bloodstream in large numbers (this is called viremia), and a second wave of multiplication occurs in the spleen, bone marrow, and lymph nodes. The initial or prodromal symptoms are similar to other viral diseases such as influenza and the common cold: fever (at least 38.5 °C (101 °F)), muscle pain, malaise, headache, prostration, and as the digestive tract is commonly involved, nausea and vomiting and backache often occur. The prodrome, or preeruptive stage, usually lasts 2–4 days. By days 12–15 the first visible lesions—small reddish spots called enanthem—appear on mucous membranes of the mouth, tongue, palate, and throat, and temperature falls to near normal. These lesions rapidly enlarge and rupture, releasing large amounts of virus into the saliva.[193]
Smallpox virus preferentially attacks skin cells, causing the characteristic pimples (called macules) associated with the disease. A rash develops on the skin 24 to 48 hours after lesions on the mucous membranes appear. Typically the macules first appear on the forehead, then rapidly spread to the whole face, proximal portions of extremities, the trunk, and lastly to distal portions of extremities. The process takes no more than 24 to 36 hours, after which no new lesions appear.[193] At this point Variola major infection can take several very different courses.
- Q fever[211]
- Leptospirosis[212]
- Bubonic plague[213]
- Meningeal plague[213]
- Pharyngeal plague[213]
- Pneumonic plague[213]
- Septicemic plague[213]
Endocarditis is an inflammation of the inner layer of the heart, the endocardium. It usually involves the heart valves (native or prosthetic valves). Other structures which may be involved include the interventricular septum, the chordae tendineae, the mural endocardium, or even on intracardiac devices. Endocarditis is characterized by a prototypic lesion, the vegetation, which is a mass of platelets, fibrin, microcolonies of microorganisms, and scant inflammatory cells.[215] In the subacute form of infective endocarditis, the vegetation may also include a center of granulomatous tissue, which may fibrose or calcify.[216]
There are multiple ways to classify endocarditis. The simplest classification is based on etiology: either infective or non-infective, depending on whether a microorganism is the source of the inflammation. Regardless, diagnosis of endocarditis is based on the clinical features, investigations such as echocardiogram, as well as any blood cultures demonstrating the presence of endocarditis-causing microorganisms.
Infective endocarditis:
Since the valves of the heart do not receive any dedicated blood supply, defensive immune mechanisms (such as white blood cells) cannot directly reach the valves via the bloodstream. If an organism (such as bacteria) attaches to a valve surface and forms a vegetation, the host immune response is blunted. The lack of blood supply to the valves also has implications on treatment,since drugs also have difficulty reaching the infected valve.
Normally, blood flows smoothly through these valves. If they have been damaged (from rheumatic fever, for example) the risk of bacteria attachment is increased.[216]
Non-infective endocarditis: Nonbacterial thrombic endocarditis (NBTE) or marantic endocarditis is most commonly found on previously undamaged valves[216]. As opposed to infective endocarditis, the vegetations in NBTE are small, sterile, and tend to aggregate along the edges of the valve or the cusps[216]. Also unlike infective endocarditis, NBTE does not cause an inflammation response from the body[216]. NBTE usually occurs during a hypercoagulable state such as system wide bacterial infection, or pregnancy, though it is also sometimes seen in patients with venous catheters[216]. NBTE may also occur in patients with cancers, particularly mucinous adenocarcinoma[216] where Trousseau syndrome can be encountered. Typically NBTE does not cause many problems on its own, but parts of the vegetations may break off and embolize to the heart or brain, or they may serve as a focus where bacteria can lodge, thus causing infective endocarditis[216].
Another form of sterile endocarditis, is termed Libman-Sacks endocarditis; this form occurs more often in patients with lupus erythematosus and is thought to be due to the deposition of immune complexes[216]. Like NBTE, Libman-Sacks endocarditis involves small vegetations, while infective endocarditis is composed of large vegetations[216]. These immune complexes precipitate an inflammation reaction, which helps to differentiate it from NBTE. Also unlike NBTE, Libman-Sacks endocarditis does not seem to have a preferred location of deposition and may form on the undersurfaces of the valves or even on the endocardium[216].
Prognosis: Features suggestive of a worse prognosis are Acute endocarditis (Staphylococcus aureus), heart failure, IV drug abuse (often left and right sided disease), prosthetic valve infection, infection of the aortic rather than mitral valve, associated rhythm disturbance. Subacute bacterial endocarditis (Streptococcus viridans) has a better prognosis.
Tuberculosis or TB (short for Tubercles Bacillus) is a common and often deadly infectious disease caused by mycobacteria, usually Mycobacterium tuberculosis in humans.[216] Tuberculosis usually attacks the lungs but can also affect other parts of the body. It is spread through the air, when people who have the disease cough, sneeze, or spit[220]. Most infections in humans result in an asymptomatic, latent infection, and about one in ten latent infections eventually progresses to active disease, which, if left untreated, kills more than 50% of its victims.
The classic symptoms are a chronic cough with blood-tinged sputum, fever, night sweats, and weight loss. Infection of other organs causes a wide range of symptoms. Diagnosis relies on radiology (commonly chest X-rays), a tuberculin skin test, blood tests, as well as microscopic examination and microbiological culture of bodily fluids. Treatment is difficult and requires long courses of multiple antibiotics. Contacts are also screened and treated if necessary. Antibiotic resistance is a growing problem in (extensively) multi-drug-resistant tuberculosis. Prevention relies on screening programs and vaccination, usually with Bacillus Calmette-Guérin vaccine.
A third of the world's population are thought to be infected with M. tuberculosis,[221] and new infections occur at a rate of about one per second.[222] The proportion of people who become sick with tuberculosis each year is stable or falling worldwide but, because of population growth, the absolute number of new cases is still increasing.[222] In 2007 there were an estimated 13.7 million chronic active cases, 9.3 million new cases, and 1.8 million deaths, mostly in developing countries.[223] In addition, more people in the developed world are contracting tuberculosis because their immune systems are compromised by immunosuppressive drugs, substance abuse, or AIDS. The distribution of tuberculosis is not uniform across the globe; about 80% of the population in many Asian and African countries test positive in tuberculin tests, while only 5-10% of the US population test positive.[216]
Classification of Tuberculosis
The current clinical classification system for tuberculosis (TB) is based on the pathogenesis of the disease.[citation needed]
| Class | Type | Description |
|---|---|---|
| 0 | No TB exposure Not infected |
No history of exposure Negative reaction to tuberculin skin test |
| 1 | TB exposure No evidence of infection |
History of exposure Negative reaction to tuberculin skin test |
| 2 | TB infection No disease |
Positive reaction to tuberculin skin test Negative bacteriologic studies (if done) No clinical, bacteriologic, or radiographic evidence of TB |
| 3 | TB, clinically active | M. tuberculosis cultured (if done) Clinical, bacteriologic, or radiographic evidence of current disease |
| 4 | TB Not clinically active |
History of episode(s) of TB or Abnormal but stable radiographic findings Positive reaction to the tuberculin skin test Negative bacteriologic studies (if done) and No clinical or radiographic evidence of current disease |
| 5 | TB suspect | Diagnosis pending TB disease should be ruled in or out within 3 months |
Signs and symptoms of Tuberculosis:
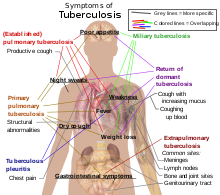


When the disease becomes active, 75% of the cases are pulmonary TB, that is, TB in the lungs. Symptoms include chest pain, coughing up blood, and a productive, prolonged cough for more than three weeks. Systemic symptoms include fever, chills, night sweats, appetite loss, weight loss, pallor, and often a tendency to fatigue very easily.[222]
In the other 25% of active cases, the infection moves from the lungs, causing other kinds of TB, collectively denoted extrapulmonary tuberculosis.[226] This occurs more commonly in immunosuppressed persons and young children. Extrapulmonary infection sites include the pleura in tuberculosis pleurisy, the central nervous system in meningitis, the lymphatic system in scrofula of the neck, the genitourinary system in urogenital tuberculosis, and bones and joints in Pott's disease of the spine. An especially serious form is disseminated TB, more commonly known as miliary tuberculosis. Extrapulmonary TB may co-exist with pulmonary TB as well.[227]
Causes of Tuberculosis:
The primary cause of TB, Mycobacterium tuberculosis, is a small aerobic non-motile bacillus. High lipid content of this pathogen accounts for many of its unique clinical characteristics.[228] It divides every 16 to 20 hours, an extremely slow rate compared with other bacteria, which usually divide in less than an hour.[229] (For example, one of the fastest-growing bacteria is a strain of E. coli that can divide roughly every 20 minutes.) Since MTB has a cell wall but lacks a phospholipid outer membrane, it is classified as a Gram-positive bacterium. However, if a Gram stain is performed, MTB either stains very weakly Gram-positive or does not retain dye due to the high lipid & mycolic acid content of its cell wall.[230] MTB can withstand weak disinfectants and survive in a dry state for weeks. In nature, the bacterium can grow only within the cells of a host organism, but M. tuberculosis can be cultured in vitro.[231]
Using histological stains on expectorate samples from phlegm (also called sputum), scientists can identify MTB under a regular microscope. Since MTB retains certain stains after being treated with acidic solution, it is classified as an acid-fast bacillus (AFB).[216][230] The most common acid-fast staining technique, the Ziehl-Neelsen stain, dyes AFBs a bright red that stands out clearly against a blue background. Other ways to visualize AFBs include an auramine-rhodamine stain and fluorescent microscopy.
The M. tuberculosis complex includes four other TB-causingmycobacteria: M. bovis, M. africanum, M. canetti and M. microti.[232] M. africanum is not widespread, but in parts of Africa it is a significant cause of tuberculosis.[233][234] M. bovis was once a common cause of tuberculosis, but the introduction of pasteurized milk has largely eliminated this as a public health problem in developed countries.[216][235] M. canetti is rare and seems to be limited to Africa, although a few cases have been seen in African emigrants.[236] M. microti is mostly seen in immunodeficient people, although it is possible that the prevalence of this pathogen has been underestimated.[237]
Other known pathogenic mycobacteria include Mycobacterium leprae, Mycobacterium avium and M. kansasii. The last two are part of the nontuberculous mycobacteria (NTM) group. Nontuberculous mycobacteria cause neither TB nor leprosy, but they do cause pulmonary diseases resembling TB.[238]
- Histoplasmosis,also known as darling's disease.[239]
- Septic arthritis[240]
- Fifth disease[241]
- Roseola[242]
- Toxic shock syndrome[243]
- Paratyphoid fever[244]
- Hepatitis A[245]
- Cat scratch disease[246]
- Respiratory syncytial virus[247]
- Echovirus[248]
- Coxsackie virus[249]
- Poliomyelitis[250]
- Rickettsialpox[251]
- Trench fever[252]
- Human Anaplasmosis[253]
- Chagas disease, often along with hepatosplenomegaly[254][255]
- Anthrax[256]
- Legionellosis[257]
- Listeria infection[258]
- Primary amebic meningoencephalitis[259]
- Rift valley fever[260]
- Hantavirus pulmonary syndrome[261]
- Lymphocytic choriomeningitis[262]
- Rat-bite fever[263]
- Rotavirus disease[264]
- Severe Acute Respiratory Syndrome[265]
- Toxoplasmosis[266]
- Leishmaniasis[267]
- Staphylococcal scalded skin syndrome[268]
- Otitis media[269]
- Malignant otitis externa[270]
- Bacterial sinusitis[271]
- Retropharyngeal abscess[272]
- Peritonsillar abscess[273]
- Epiglottitis[274]
- Rabies[275]
- Venezuelan equine encephalitis virus[276]
- Herpes simplex encephalitis
- Dental abscess[277]
- Brain abscess[278]
- Croup[279]
- Pyelonephritis[280]
- Cystitis[281]
- Cholangitis[282]
- Hepatic abscess[283]
- Orbital cellulitis[284]
References
[edit]- ^ "Diphtheria: eMedicine Emergency Medicine". Emedicine.medscape.com. 2009-10-05. Retrieved 2010-03-09.
- ^ Atkinson W, Hamborsky J, McIntyre L, Wolfe S, eds. (2007). Diphtheria. in: Epidemiology and Prevention of Vaccine-Preventable Diseases (The Pink Book) (PDF) (10 ed.). Washington DC: Public Health Foundation. pp. 59–70.
{{cite book}}:|author=has generic name (help)CS1 maint: multiple names: authors list (link) - ^ https://health.google.com/health/ref/Tetanus
- ^ a b c d Wells CL, Wilkins TD (1996). "Clostridia: Sporeforming Anaerobic Bacilli". In Baron S, et alublisher = Univ of Texas Medical Branch (ed.). Baron's Medical Microbiology. ISBN 0-9631172-1-1. Cite error: The named reference "Baron" was defined multiple times with different content (see the help page).
- ^ "Tetanus" (PDF). CDC Pink Book. Retrieved 2007-01-26.
- ^ World Health Organization (2000-11-01). "Maternal and Neonatal Tetanus Elimination by 2005" (PDF). Retrieved 2007-01-26.
- ^ https://health.google.com/health/ref/Pertussis
- ^ "WHO | Influenza". Who.int. 2009-11-13. Retrieved 2010-03-09.
- ^ a b "Influenza: Viral Infections: Merck Manual Home Edition". www.merck.com. Retrieved 2008-03-15.
- ^ Eccles, R (2005). "Understanding the symptoms of the common cold and influenza". Lancet Infect Dis. 5 (11): 718–25. doi:10.1016/S1473-3099(05)70270-X. PMID 16253889.
- ^ Seasonal Flu vs. Stomach Flu by Kristina Duda, R.N.; accessed 12 March 2007 (Website: "About, Inc., A part of The New York Times Company")
- ^ Cite error: The named reference
Brankstonwas invoked but never defined (see the help page). - ^ Suarez, D. L.; Spackman, E.; Senne, D. A.; Bulaga, L.; Welsch, A. C.; Froberg, K. (2003). "The effect of various disinfectants on detection of avian influenza virus by real time RT-PCR". Avian Dis. 47 (3 Suppl): 1091–5. doi:10.1637/0005-2086-47.s3.1091. PMID 14575118.
{{cite journal}}: CS1 maint: date and year (link) - ^ Avian Influenza (Bird Flu): Implications for Human Disease. Physical characteristics of influenza A viruses. UMN CIDRAP.
- ^ Influenza (Seasonal), World Heath Organization, April 2009, retrieved 2010-02-13
- ^ Jonathan Dushoff; Plotkin, JB; Viboud, C; Earn, DJ; Simonsen, L (2006). "Mortality due to Influenza in the United States — An Annualized Regression Approach Using Multiple-Cause Mortality Data". American Journal of Epidemiology. 163 (2): 181–7. doi:10.1093/aje/kwj024. PMID 16319291. Retrieved 2009-10-29.
The regression model attributes an annual average of 41,400 (95% confidence interval: 27,100, 55,700) deaths to influenza over the period 1979–2001.
- ^ "Avian influenza ("bird flu") fact sheet". WHO. February 2006. Retrieved 2006-10-20.
{{cite web}}: CS1 maint: date and year (link) - ^ World Health Organization. World now at the start of 2009 influenza pandemic. http://www.who.int/mediacentre/news/statements/2009/h1n1_pandemic_phase6_20090611/en/index.html
- ^ WHO position paper: influenza vaccines WHO weekly Epidemiological Record 19 August 2005, vol. 80, 33, pp. 277–288.
- ^ Villegas, P (1998). "Viral diseases of the respiratory system". Poult Sci. 77 (8): 1143–5. doi:10.1093/ps/77.8.1143. PMID 9706079.
- ^ Horwood F, Macfarlane J (October 2002). "Pneumococcal and influenza vaccination: current situation and future prospects". Thorax. 57 (Suppl 2): II24–II30. PMC 1766003. PMID 12364707.
{{cite journal}}: CS1 maint: date and year (link) - ^ "Mononucleosis: Symptoms". MayoClinic.com. Retrieved 2010-03-09.
- ^ a b synd/1811 at Who Named It?
- ^ http://www.kenyon.edu/x26163.xml
- ^ "Infectious Mononucleosis (mono, EBV mononucleosis)". Health.state.ny.us. Retrieved 2009-11-27.
- ^ Н. Филатов: Лекции об острых инфекционных болезнях у детей (N. Filatov: Lektsii ob ostrikh infeksionnîkh boleznyakh u dietei). 2 volumes. Moscow, A. Lang, 1887.
- ^ E. Pfeiffer: Drüsenfieber. Jahrbuch für Kinderheilkunde und physische Erziehung, Wien, 1889, 29: 257-264.
- ^ WebMD > Infectious Mononucleosis Last Updated: September 19, 2007. Retrieved on 7 Mars, 2009
- ^ (History section of) eMedicine Specialties > Infectious Diseases > Infectious Mononucleosis. Author: Burke A Cunha, MD, Professor of Medicine
- ^ Longmore, Murray (2007). Oxford Handbook of Clinical Medicine, 7th edition. Oxford University Press. p. 389. ISBN 978-0-19-856837-7.
{{cite book}}: Unknown parameter|coauthors=ignored (|author=suggested) (help) - ^ Ebell MH (November 2004). "Epstein-Barr virus infectious mononucleosis". American Family Physician. 70 (7): 1279–87. PMID 15508538.
{{cite journal}}: CS1 maint: date and year (link) - ^ "WHO | Ebola haemorrhagic fever". Who.int. Retrieved 2010-03-09.
- ^ Bardi, Jason Socrates (2002). "Death Called a River". Scripps Research Institute. 2 (1). Retrieved 2006-12-08.
- ^ Netesov, SV; Feldmann, H; Jahrling, PB; Kiley, MP; Klenk, H-D; Sanchez, A (2004-04-24). "Filoviridae". International Committee on Taxonomy of Viruses. Retrieved 2009-10-04.
- ^ Büchen-Osmond, Cornelia (2006-04-25). "ICTVdB Virus Description - 01.025.0.02. Ebolavirus". International Committee on Taxonomy of Viruses. Retrieved 2009-06-02.
- ^ Suzuki, Y; Gojobori (1997). "The origin and evolution of Ebola and Marburg viruses". Molecular Biology and Evolution. 14 (8): 800–6. doi:10.1093/oxfordjournals.molbev.a025820. PMID 9254917.
- ^ Isaacson, M; Sureau, P; Courteille, G; Pattyn, SR. "Clinical Aspects of Ebola Virus Disease at the Ngaliema Hospital, Kinshasa, Zaire, 1976". Retrieved 2009-07-08.
{{cite journal}}: Cite journal requires|journal=(help)CS1 maint: multiple names: authors list (link) - ^ a b Special Pathogens Branch CDC (2008-01-14). "Known Cases and Outbreaks of Ebola Hemorrhagic Fever". Center for Disease Control and Prevention. Retrieved 2008-08-02.
- ^ "Pig-to-Human Ebola Case Suspected in Philippines". New York Times. 2009-01-24. Retrieved 2009-01-26.
- ^ McCormick & Fisher-Hoch 1999, p. 300
- ^ Waterman, Tara (1999). Ebola Cote D'Ivoire Outbreaks. Stanford University. Retrieved 2009-05-30.
- ^ "End of Ebola outbreak in Uganda" (Press release). World Health Organization. 2008-02-20.
- ^ Cocks, Tim (2007-12-11). "Uganda confirms 113 suspected Ebola cases". Reuters. Retrieved 2008-02-25.
- ^ Klenk & Feldmann 2004, p. 9
- ^ Cite error: The named reference
BiomarkerDatabasewas invoked but never defined (see the help page). - ^ Sullivan, N.; Yang, Z. -Y.; Nabel, G. J. (2003). "Ebola Virus Pathogenesis: Implications for Vaccines and Therapies" (Free full text). Journal of Virology. 77 (18): 9733–9737. doi:10.1128/JVI.77.18.9733-9737.2003. PMC 224575. PMID 12941881.
- ^ a b c Pourrut, X.; Kumulungui, B.; Wittmann, T.; Moussavou, G.; Délicat, A.; Yaba, P.; Nkoghe, D.; Gonzalez, J. P.; Leroy, E. M. (2005). "The natural history of Ebola virus in Africa". Microbes and Infection / Institut Pasteur. 7 (7–8): 1005–1014. doi:10.1016/j.micinf.2005.04.006. PMID 16002313.
- ^ Morvan, J.; Deubel, V.; Gounon, P.; Nakouné, E.; Barrière, P.; Murri, S.; Perpète, O.; Selekon, B.; Coudrier, D.; Gautier-Hion, A.; Colyn, M.; Volehkov, V. (1999). "Identification of Ebola virus sequences present as RNA or DNA in organs of terrestrial small mammals of the Central African Republic". Microbes and Infection. 1 (14): 1193–1201. doi:10.1016/S1286-4579(99)00242-7. PMID 10580275.
- ^ "Fruit bats may carry Ebola virus". BBC News. 2005-12-11. Retrieved 2008-02-25.
- ^ Swanepoel, R. L.; Leman, P. A.; Burt, F. J.; Zachariades, N. A.; Braack, L. E.; Ksiazek, T. G.; Rollin, P. E.; Zaki, S. R.; Peters, C. J. (Oct 1996). "Experimental inoculation of plants and animals with Ebola virus". Emerging Infectious Diseases. 2 (4): 321–325. doi:10.3201/eid0204.960407. ISSN 1080-6040. PMC 2639914. PMID 8969248.
- ^ Leroy, E. M.; Kumulungui, B.; Pourrut, X.; Rouquet, P.; Hassanin, A.; Yaba, P.; Délicat, A.; Paweska, J. T.; Gonzalez, J. P.; Swanepoel, R. (2005). "Fruit bats as reservoirs of Ebola virus". Nature. 438 (7068): 575–576. Bibcode:2005Natur.438..575L. doi:10.1038/438575a. PMID 16319873.
- ^ Pourrut, X.; Délicat, A.; Rollin, P. E.; Ksiazek, T. G.; Gonzalez, J.‐P.; Leroy, E. M. (2007). "Spatial and temporal patterns of Zaire ebolavirus antibody prevalence in the possible reservoir bat species". The Journal of Infectious Diseases. Suppl 2 (s2): S176–S183. doi:10.1086/520541. PMID 17940947.
- ^ Lubroth, Juan. "Ebola-Reston Virus in Pigs: Disease situation in swine in the Philippines". Food and Agriculture Organization of the United Nations. Retrieved 2009-09-27.
- ^ Gonzalez, J. P.; Pourrut, X.; Leroy, E. (2007). "Ebolavirus and other filoviruses". Current Topics in Microbiology and Immunology. 315: 363–387. doi:10.1007/978-3-540-70962-6_15. ISBN 978-3-540-70961-9. PMID 17848072.
{{cite journal}}: Cite journal requires|journal=(help) - ^ Peterson, A. T.; Bauer, J. T.; Mills, J. N. (2004). "Ecologic and Geographic Distribution of Filovirus Disease". Emerging Infectious Diseases. 10 (1): 40–47. doi:10.3201/eid1001.030125. PMC 3322747. PMID 15078595.
- ^ Questions and Answers about Ebola Hemorrhagic Fever. Centers for Disease Control and Prevention. 2009-03-25. Retrieved 2009-05-31.
- ^ Jaax, N. J.; Jahrling, P.; Geisbert, T.; Geisbert, J.; Steele, K.; McKee, K.; Nagley, D.; Johnson, E.; Jaax, G.; Peters, C. (Dec 1995). "Transmission of Ebola virus (Zaire strain) to uninfected control monkeys in a biocontainment laboratory". Lancet. 346 (8991–8992): 1669–1671. doi:10.1016/S0140-6736(95)92841-3. ISSN 0140-6736. PMID 8551825.
- ^ Jaax, N. D.; Davis, K. J.; Geisbert, T. J.; Vogel, P.; Jaax, G. P.; Topper, M.; Jahrling, P. B. (Feb 1996). "Timed appearance of lymphocytic choriomeningitis virus after gastric inoculation of mice". Archives of Pathology & Laboratory Medicine. 120 (2): 140–155. ISSN 0003-9985. PMID 8712894.
- ^ Johnson, E. J.; Jaax, N.; White, J.; Jahrling, P. (Aug 1995). "Lethal experimental infections of rhesus monkeys by aerosolized Ebola virus". International Journal of Experimental Pathology. 76 (4): 227–236. ISSN 0959-9673. PMC 1997182. PMID 7547435.
- ^ Leffel, E. R.; Reed, D. S. (2004). "Marburg and Ebola viruses as aerosol threats". Biosecurity and Bioterrorism : Biodefense Strategy, Practice, and Science. 2 (3): 186–191. doi:10.1089/bsp.2004.2.186. ISSN 1538-7135. PMID 15588056.
- ^ Harden, Blaine (2001-02-18). "Dr. Matthew's Passion". New York Times Magazine. Retrieved 2008-02-25.
- ^ Centers for Disease Control and Prevention and World Health Organization (1998). Infection Control for Viral Haemorrhagic Fevers in the African Health Care Setting (PDF). Atlanta, Georgia, USA: Centers for Disease Control and Prevention. Retrieved 2009-05-31.
- ^ Sullivan, J.; Geisbert, W.; Geisbert, B.; Xu, L.; Yang, Y.; Roederer, M.; Koup, A.; Jahrling, B.; Nabel, J. (2003). "Accelerated vaccination for Ebola virus haemorrhagic fever in non-human primates". Nature. 424 (6949): 681–684. doi:10.1038/nature01876. PMID 12904795.
- ^ Jones, M.; Feldmann, H.; Ströher, U.; Geisbert, J. B.; Fernando, L.; Grolla, A.; Klenk, H. D.; Sullivan, N. J.; Volchkov, V. E.; Fritz, E. A.; Daddario, K. M.; Hensley, L. E.; Jahrling, P. B.; Geisbert, T. W. (2005). "Live attenuated recombinant vaccine protects nonhuman primates against Ebola and Marburg viruses". Nature Medicine. 11 (7): 786–790. doi:10.1038/nm1258. PMID 15937495.
- ^ Oplinger, Anne A. (2003-11-18). NIAID Ebola vaccine enters human trial. Bio-Medicine.
- ^ "Ebola/Marburg Vaccine Development" (Press release). National Institute of Allergy and Infectious Diseases. 2008-09-15.
- ^ Francesconi, P.; Yoti, Z.; Declich, S.; Onek, P. A.; Fabiani, M.; Olango, J.; Andraghetti, R.; Rollin, P. E.; Opira, C.; Greco, D.; Salmaso, S. (2003). "Ebola Hemorrhagic Fever Transmission and Risk Factors of Contacts, Uganda" (Free full text). Emerging Infectious Diseases. 9 (11): 1430–1437. doi:10.3201/eid0911.030339. PMC 3035551. PMID 14718087.
- ^ "End of Ebola outbreak in Uganda" (Press release). World Health Organization. 2008-02-20. Retrieved 2009-11-29.
- ^ Cite error: The named reference
10.1371/journal.pmed.0010059was invoked but never defined (see the help page). - ^ Peters, C. J.; Khan, A. S. (1999). "Filovirus diseases". Current Topics in Microbiology and Immunology. 235: 85–95. doi:10.1007/978-3-642-59949-1_6. ISBN 978-3-642-64193-0. PMID 9893380.
{{cite journal}}: Cite journal requires|journal=(help) - ^ Zaki, S. R.; Kilmarx, P. (1997). "Ebola Virus Hemorrhagic Fever". Pathology of Emerging Infections. Washington, DC: American Society for Microbiology.
- ^ "Marburg Hemorrhagic Fever: Q & A | CDC Special Pathogens Branch". Cdc.gov. Retrieved 2010-03-09.
- ^ Büchen-Osmond, Cornelia (2006-04-25). "ICTVdB Virus Description - 01.025.0.01. Marburgvirus". International Committee on Taxonomy of Viruses. Retrieved 2009-02-09.
- ^ [1]
- ^ Mackenzie, Debora (2007-09-01), "Marburg virus found in fruit bats.", New Scientist, no. 2619, p. 14, retrieved 2007-09-26
- ^ Towner JS, Pourrut X, Albariño CG; et al. (2007). "Marburg virus infection detected in a common African bat". PLOS ONE. 2 (1): e764. doi:10.1371/journal.pone.0000764. PMC 1942080. PMID 17712412.
{{cite journal}}: Explicit use of et al. in:|author=(help)CS1 maint: multiple names: authors list (link) - ^ "CDC special pathogins branch- Marburg page". Retrieved 2007-05-03.
- ^ "World Health Organization - Report after final death 2004-2005 outbreak". Retrieved 2007-05-03.
- ^ "Marburg virus - Material Safety Data Sheets (MSDS)". Public Health Agency of Canada. 1997-10-11. Retrieved 2008-10-12.
- ^ Hevey, M.; Negley, D.; Pushko, P.; Smith, J.; Schmaljohn, A. (Nov 1998). "Marburg virus vaccines based upon alphavirus replicons protect guinea pigs and nonhuman primates". Virology. 251 (1): 28–37. doi:10.1006/viro.1998.9367. ISSN 0042-6822. PMID 9813200.
- ^ Jones SM, Feldmann H, Stroher U; et al. (2005). "Live attenuated recombinant vaccine protects nonhuman primates against Ebola and Marburg viruses". Nature Med. 11 (7): 786–90. doi:10.1038/nm1258. PMID 15937495.
{{cite journal}}: Explicit use of et al. in:|author=(help)CS1 maint: multiple names: authors list (link) - ^ Daddario-DiCaprio KM, Geisbert TW, Ströher U; et al. (2006). "Postexposure protection against Marburg haemorrhagic fever with recombinant vesicular stomatitis virus vectors in non-human primates: an efficacy assessment". Lancet. 367 (9520): 1399–404. doi:10.1016/S0140-6736(06)68546-2. PMID 16650649.
{{cite journal}}: Explicit use of et al. in:|author=(help)CS1 maint: multiple names: authors list (link) - ^ [2]
- ^ "Strep throat: MedlinePlus Medical Encyclopedia". Nlm.nih.gov. 2010-02-23. Retrieved 2010-03-09.
- ^ "streptococcal pharyngitis" at Dorland's Medical Dictionary
- ^ Xu J, Schwartz K, Monsur J, Northrup J, Neale AV (December 2004). "Patient-clinician agreement on signs and symptoms of 'strep throat': a MetroNet study". Fam Pract. 21 (6): 599–604. doi:10.1093/fampra/cmh604. PMID 15528291.
{{cite journal}}: CS1 maint: date and year (link) CS1 maint: multiple names: authors list (link) - ^ Know the Tell-Tale Signs of Strep, http://www.myfamilywellness.org/MainMenuCategories/FamilyHealthCenter/ChildrensHealth/Strep.aspx
- ^ Kids Health
- ^ a b c Bisno AL, Gerber MA, Gwaltney JM, Kaplan EL, Schwartz RH (July 2002). "Practice guidelines for the diagnosis and management of group A streptococcal pharyngitis. [[Infectious Diseases Society of America]]". Clin. Infect. Dis. 35 (2): 113–25. doi:10.1086/340949. PMID 12087516.
{{cite journal}}: URL–wikilink conflict (help)CS1 maint: date and year (link) CS1 maint: multiple names: authors list (link) - ^ https://health.google.com/health/ref/Pneumonia
- ^ pneumonia at eMedicine Dictionary
- ^ "pneumonia" at Dorland's Medical Dictionary
- ^ World Health Organization. Global causes of under 5 mortality. http://www.who.int/entity/child_adolescent_health/media/causes_death_u5_neonates_2004.pdf.
- ^ Feldman C, Kallenbach JM, Levy H, Thorburn JR, Hurwitz MD, Koornhof HJ (1991). "Comparison of bacteraemic community-acquired lobar pneumonia due to Streptococcus pneumoniae and Klebsiella pneumoniae in an intensive care unit". Respiration. 58 (5–6): 265–70. doi:10.1159/000195943. PMID 1792415.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ Table 13-7 in: Mitchell, Richard Sheppard; Kumar, Vinay; Abbas, Abul K.; Fausto, Nelson (2007). Robbins Basic Pathology (8th ed.). Philadelphia: Saunders. ISBN 978-1-4160-2973-1.
{{cite book}}: CS1 maint: multiple names: authors list (link) - ^ "UpToDate Inc". Retrieved ~~~~~.
{{cite web}}: Check date values in:|accessdate=(help) - ^ Krause DC, Balish MF (February 2004). "Cellular engineering in a minimal microbe: structure and assembly of the terminal organelle of Mycoplasma pneumoniae". Mol. Microbiol. 51 (4): 917–24. doi:10.1046/j.1365-2958.2003.03899.x. PMID 14763969.
{{cite journal}}: CS1 maint: date and year (link) - ^ "Septicemia: MedlinePlus Medical Encyclopedia". Nlm.nih.gov. Retrieved 2010-03-09.
- ^ Levy MM, Fink MP, Marshall JC; et al. (April 2003). "2001 SCCM/ESICM/ACCP/ATS/SIS International Sepsis Definitions Conference". Crit. Care Med. 31 (4): 1250–6. doi:10.1097/01.CCM.0000050454.01978.3B. PMID 12682500.
{{cite journal}}: Explicit use of et al. in:|author=(help)CS1 maint: date and year (link) CS1 maint: multiple names: authors list (link) - ^ a b c Bone RC, Balk RA, Cerra FB; et al. (Jun 1992). "Definitions for sepsis and organ failure and guidelines for the use of innovative therapies in sepsis. The ACCP/SCCM Consensus Conference Committee. American College of Chest Physicians/Society of Critical Care Medicine". Chest. 101 (6): 1644–55. doi:10.1378/chest.101.6.1644. PMID 1303622.
{{cite journal}}: Explicit use of et al. in:|author=(help)CS1 maint: date and year (link) CS1 maint: multiple names: authors list (link) - ^ Dictionary
- ^ septicemia at eMedicine Dictionary
- ^ Dellinger RP, Levy MM, Carlet JM, et al., for the International Surviving Sepsis Campaign Guidelines Committee. (2008). "Surviving Sepsis Campaign: International Guidelines for Management of Severe Sepsis and Septic Shock: 2008" (Subscription required). Crit Care Med. 36 (1): 296–327. doi:10.1097/01.CCM.0000298158.12101.41. PMID 18158437.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ Lockhart PB, Brennan MT, Sasser HC, Fox PC, Paster BJ, Bahrani-Mougeot FK (Jun 2008). "Bacteremia associated with toothbrushing and dental extraction". Circulation. 117 (24): 3118–25. doi:10.1161/CIRCULATIONAHA.107.758524. PMID 18541739.
{{cite journal}}: CS1 maint: date and year (link) CS1 maint: multiple names: authors list (link) - ^ Wilson W, Taubert KA, Gewitz M; et al. (Oct 2007). "Prevention of infective endocarditis: guidelines from the American Heart Association: a guideline from the American Heart Association Rheumatic Fever, Endocarditis, and Kawasaki Disease Committee, Council on Cardiovascular Disease in the Young, and the Council on Clinical Cardiology, Council on Cardiovascular Surgery and Anesthesia, and the Quality of Care and Outcomes Research Interdisciplinary Working Group". Circulation. 116 (15): 1736–54. doi:10.1161/CIRCULATIONAHA.106.183095. PMID 17446442.
{{cite journal}}: Explicit use of et al. in:|author=(help)CS1 maint: date and year (link) CS1 maint: multiple names: authors list (link) - ^ "Malaria: Symptoms". MayoClinic.com. Retrieved 2010-03-09.
- ^ Malaria Facts. Centers for Disease Control and Prevention.
- ^ Snow RW, Guerra CA, Noor AM, Myint HY, Hay SI (2005). "The global distribution of clinical episodes of Plasmodium falciparum malaria". Nature. 434 (7030): 214–7. doi:10.1038/nature03342. PMID 15759000.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ "Malaria: Disease Impacts and Long-Run Income Differences" (PDF). Institute for the Study of Labor. Retrieved 2008-12-10.
- ^ Fong YL, Cadigan FC, Coatney GR (1971). "A presumptive case of naturally occurring Plasmodium knowlesi malaria in man in Malaysia". Trans. R. Soc. Trop. Med. Hyg. 65 (6): 839–40. doi:10.1016/0035-9203(71)90103-9. PMID 5003320.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ Singh B, Kim Sung L, Matusop A; et al. (March 2004). "A large focus of naturally acquired Plasmodium knowlesi infections in human beings". Lancet. 363 (9414): 1017–24. doi:10.1016/S0140-6736(04)15836-4. PMID 15051281.
{{cite journal}}: Explicit use of et al. in:|author=(help)CS1 maint: date and year (link) CS1 maint: multiple names: authors list (link) - ^ Dondorp AM, Day NP (July 2007). "The treatment of severe malaria". Trans. R. Soc. Trop. Med. Hyg. 101 (7): 633–4. doi:10.1016/j.trstmh.2007.03.011. PMID 17434195.
{{cite journal}}: CS1 maint: date and year (link) - ^ Wellems TE (October 2002). "Plasmodium chloroquine resistance and the search for a replacement antimalarial drug". Science. 298 (5591): 124–6. doi:10.1126/science.1078167. PMID 12364789.
{{cite journal}}: CS1 maint: date and year (link) - ^ Kilama W, Ntoumi F (October 2009). "Malaria: a research agenda for the eradication era". Lancet. 374 (9700): 1480–2. doi:10.1016/S0140-6736(09)61884-5. PMID 19880004.
{{cite journal}}: CS1 maint: date and year (link) - ^ "Chapter 4, Prevention of Specific Infectious Diseases". CDC Traveler's Health: Yellow Book. Retrieved 2007-05-20.
- ^ "Dengue fever outbreak in Bolivia". BBC. 2009-02-03. Retrieved 2009-02-26.
- ^ Reuters, "Dengue Fever Hits Paraguay", New York Times" (March 4, 2007)
- ^ http://www.samoalivenews.com/Health/Dengue-Fever-Outbreak-Confirmed-In-Samoa.html
- ^ "Zhuhai reports outbreak of dengue fever". China Daily. 200-09-06. Retrieved 2009-11-18.
{{cite web}}: Check date values in:|date=(help) - ^ Dengue Fever – Information Sheet. World Health Organization, October 9, 2006. Retrieved on 2007-11-30.
- ^ Dengue epidemic threatens India's capital
- ^ a b c d Ryan KJ, Ray CG (editors) (2004). Sherris Medical Microbiology (4th ed.). McGraw Hill. p. 592. ISBN 0838585299.
{{cite book}}:|author=has generic name (help) Cite error: The named reference "Sherris" was defined multiple times with different content (see the help page). - ^ Such a diagnostic kit may consist of a "test cassette" or other device. One type is described as follows: "Dengue fever rapid test devices, also known as one-step dengue tests, are a solid phase immuno-chromatographic assay for the rapid, qualitative and differential detection of dengue IgG and IgM antibodies to dengue fever virus in human serum, plasma or whole blood." Atlas Link Biotech co. Ltd (2008)Dengue Fever Rapid Test Kits. Accessed: 27/06/09. Available at: http://www.ivdpretest.com/Dengue-Rapid-Tests.html
- ^ Hanley, K.A. and Weaver, S.C. (editors) (2010). Frontiers in Dengue Virus Research. Caister Academic Press. ISBN 978-1-904455-50-9.
{{cite book}}:|author=has generic name (help)CS1 maint: multiple names: authors list (link) - ^ "WHO | Meningococcal meningitis". Who.int. 2010-02-15. Retrieved 2010-03-09.
- ^ Meningococcal Disease (2001) Humana Press, Andrew J. Pollard and Martin C.J. Maiden
- ^ Reido, F.S. et al. 1995. Ped. Infect. Dis. J. 14, PP. 643–657
- ^ Centers for Disease Control and Prevention. Meningococcal disease among college students: ACIP modifies recommendations for meningitis vaccination. Press release. 1999 Oct 20
- ^ Jeeri R. Reddy and Thiombiano S. Rigobert. Infections à méningocoques Maladies infectieuses et Africa. West Africa. Med. Bull. 2007
- ^ Jeeri R Reddy and Thiombiano S. Rigobert. Infections à méningocoques Maladies infectieuses et Africa. West Africa. Med. Bull. 2008
- ^ WHO/EMC/BAC/98.3
- ^ "Hantavirus Pulmonary Syndrome in Five Pediatric Patients - Four States, 2009". Cdc.gov. Retrieved 2010-03-09.
- ^ Schudel, Matt (2007-12-24). "Terry Yates, 57; biologist found source of hantavirus". Washington Post. Boston Globe. Retrieved 2007-01-04.
- ^ "Scarlet Fever". Kidshealth.org. Retrieved 2010-03-09.
- ^ "West Nile Encephalitis: eMedicine Infectious Diseases". Emedicine.medscape.com. 2008-08-01. Retrieved 2010-03-09.
- ^ James, William D.; Berger, Timothy G.; et al. (2006). Andrews' Diseases of the Skin: clinical Dermatology. Saunders Elsevier. ISBN 0-7216-2921-0.
{{cite book}}: Explicit use of et al. in:|author=(help)CS1 maint: multiple names: authors list (link) - ^ wrongdiagnosis.com skin rash causes
- ^
Kahn, J. O. and Walker, B. D. (1998). "Acute Human Immunodeficiency Virus type 1 infection". N. Engl. J. Med. 331 (1): 33–39. doi:10.1056/NEJM199807023390107. PMID 9647878.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ Serrano, P.; Hernandez, N.; Arroyo, J. A.; Llobet, J. M. d.; Domingo, P. (2007). "Bilateral Bell Palsy and Acute HIV Type 1 Infection: Report of 2 Cases and Review". Clinical Infectious Diseases. 44 (6): e57–e61. doi:10.1086/511876. PMID 17304442.
- ^ Daar ES, Little S, Pitt J; et al. (2001). "Diagnosis of primary HIV-1 infection. Los Angeles County Primary HIV Infection Recruitment Network". Ann. Intern. Med. 134 (1): 25–9. doi:10.7326/0003-4819-134-1-200101020-00010. PMID 11187417.
{{cite journal}}: Explicit use of et al. in:|author=(help)CS1 maint: multiple names: authors list (link) - ^ Rapini, Ronald P.; Bolognia, Jean L.; Jorizzo, Joseph L. (2007). Dermatology: 2-Volume Set. St. Louis: Mosby. ISBN 978-1-4160-2999-1.
{{cite book}}: CS1 maint: multiple names: authors list (link) - ^ Hu MD, Linden (2009). "Clinical Manifestations of Lyme Disease in Adults". UpToDate. UpToDate.
- ^ Cairns V, Godwin J (2005). "Post-Lyme borreliosis syndrome: a meta-analysis of reported symptoms". Int J Epidemiol. 34 (6): 1340–1345. doi:10.1093/ije/dyi129. PMID 16040645.
- ^ Cite error: The named reference
Singhwas invoked but never defined (see the help page). - ^ "Learn More About Lyme Disease". Webmd.com. Retrieved 2010-03-09.
- ^ "Varicella: eMedicine Pediatrics: General Medicine". Emedicine.medscape.com. 2009-07-31. Retrieved 2010-03-09.
- ^ Wood MJ (October 2000). "History of Varicella Zoster Virus". Herpes. 7 (3): 60–65. PMID 11867004.
{{cite journal}}: CS1 maint: date and year (link) - ^ http://stroke.ahajournals.org/cgi/content/abstract/32/6/1257
- ^ "Measles: MedlinePlus". Nlm.nih.gov. Retrieved 2010-03-09.
- ^ "Measles".
- ^ Merriam-webster:Rubeola Accessed 2009-09-20.
- ^ T. E. C. Jr. Letters to the editor Pediatrics Vol. 49 No. 1 January 1972, pp. 150-151.
- ^ Webster's Online Dictionary: German measles Accessed 2009-09-20
- ^ "Mumps Causes, Symptoms, Treatment, MMR Vaccine and Prevention Facts on". Medicinenet.com. Retrieved 2010-03-09.
- ^ https://health.google.com/health/ref/Rubella
- ^ "Common Cold". Kidshealth.org. Retrieved 2010-03-09.
- ^ http://www.cdc.gov/EasternEquineEncephalitis/index.html
- ^ http://mayoclinic.com/health/viral-gastroenteritis/DS00085/DSECTION=symptoms
- ^ http://www.mayoclinic.com/health/rocky-mountain-spotted-fever/DS00600/DSECTION=symptoms
- ^ http://mayoclinic.com/health/appendicitis/DS00274/DSECTION=symptoms,
- ^ "Schistosomiasis". Webmd.com. Retrieved 2010-03-09.
- ^ "Meningitis- What is Meningitis? - Meningitis Overview". Children.webmd.com. 2008-12-24. Retrieved 2010-03-09.
- ^ "CDC Tularemia | Key Facts About Tularemia". Bt.cdc.gov. 2003-10-07. Retrieved 2010-03-09.
- ^ "Typhus: eMedicine Infectious Diseases". Emedicine.medscape.com. 2008-09-18. Retrieved 2010-03-09.
- ^ "Home - CDC Tick Borne Relapsing Fever". Cdc.gov. Retrieved 2010-03-09.
- ^ "Typhoid fever: Symptoms". MayoClinic.com. Retrieved 2010-03-09.
- ^ MedlinePlus Encyclopedia: Typhoid fever
- ^ CDC Disease Info typhoidfever_g
- ^ "Peritonitis". Umm.edu. 2008-09-16. Retrieved 2010-03-09.
- ^ "Biology Online's definition of peritonism". Retrieved 2008-08-14.
- ^ "Necrotizing Fasciitis: eMedicine Emergency Medicine". Emedicine.medscape.com. 2009-03-25. Retrieved 2010-03-09.
- ^ K.S. Kotrappa, R.S. Bansal, N.M. Amin, "Necrotizing fasciitis", Am Fam Physician, 1996 Apr;53(5):1691-7.
- ^ Lee TC, Carrick MM, Scott BG; et al. (2007). "Incidence and clinical characteristics of methicillin-resistant fasciitis in a large urban hospital". Am J Surg. 194 (6): 809–13. doi:10.1016/j.amjsurg.2007.08.047. PMID 18005776.
{{cite journal}}: Explicit use of et al. in:|author=(help)CS1 maint: multiple names: authors list (link) - ^ http://www.medscape.com/viewarticle/444061
- ^ Necrotizing Fasciitis (Flesh-Eating Bacteria)
- ^ "cellulitis" at Dorland's Medical Dictionary
- ^ HS-USA :: What is Hidradenitis Suppurativa?". http://www.hs-usa.org/hidradenitis_suppurativa.htm. Retrieved 2007-07-08
- ^ ClinicalTrials.gov NCT00329823 Etanercept in Hidradenitis Suppurativa
- ^ DermNet acne/hidradenitis-suppurativa
- ^ "HSF - What is Hidradenitis Suppurativa? What is HS?". http://www.hs-foundation.org/abouths/what.htm. Retrieved 2007-07-08.
- ^ "Yellow Fever: eMedicine Infectious Diseases". Emedicine.medscape.com. Retrieved 2010-03-09.
- ^ "CDC Yellow Fever". Retrieved 2010-03-13.
- ^ Oldstone, Michael B. A. (2000). Viruses, Plagues, and History (1st ed.). Oxford University Press. p. 45. ISBN 0195134222.
- ^ "Yellow fever fact sheet". WHO—Yellow fever. Retrieved 2006-04-18.
- ^ Cite error: The named reference
pmid19327647was invoked but never defined (see the help page). - ^ Barrett AD, Higgs S (2007). "Yellow fever: a disease that has yet to be conquered". Annu. Rev. Entomol. 52: 209–29. doi:10.1146/annurev.ento.52.110405.091454. PMID 16913829.
- ^ "WHO | Yellow fever". Retrieved 2009-08-13.
- ^ Chastel C (August 2003). "[Centenary of the discovery of yellow fever virus and its transmission by a mosquito (Cuba 1900–1901)]". Bull Soc Pathol Exot (in French). 96 (3): 250–6. PMID 14582304.
{{cite journal}}: CS1 maint: date and year (link) - ^ Monath TP (April 2008). "Treatment of yellow fever". Antiviral Res. 78 (1): 116–24. doi:10.1016/j.antiviral.2007.10.009. PMID 18061688.
{{cite journal}}: CS1 maint: date and year (link) - ^ "CDC Smallpox Q & A". Bt.cdc.gov. 2009-03-13. Retrieved 2010-03-09.
- ^ a b Barquet N, Domingo P (15 October 1997). "Smallpox: the triumph over the most terrible of the ministers of death". Ann. Intern. Med. 127 (8 Pt 1): 635–42. doi:10.7326/0003-4819-127-8_Part_1-199710150-00010. PMID 9341063.
- ^ a b Behbehani AM (1 December 1983). "The smallpox story: life and death of an old disease". Microbiol Rev. 47 (4): 455–509. doi:10.1128/mr.47.4.455-509.1983. PMC 281588. PMID 6319980.
- ^ a b c d e f "Smallpox". Armed Forces Institute of Pathology: Department of Infectious and Parasitic Diseases. Archived from the original on 2007-10-09. Retrieved 2008-10-28.
- ^ Jezek Z, Hardjotanojo W, Rangaraj AG (1981). "Facial scarring after varicella. A comparison with variola major and variola minor". Am. J. Epidemiol. 114 (6): 798–803. doi:10.1093/oxfordjournals.aje.a113250. PMID 7315828.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ "Epidemics and pandemics: their impacts on human history". J. N. Hays (2005). p.151. ISBN 1851096582
- ^ "Smallpox and Vaccinia". National Center for Biotechnology Information.
- ^ Riedel S (2005). "Edward Jenner and the history of smallpox and vaccination". Proc (Bayl Univ Med Cent). 18 (1): 21–5. PMC 1200696. PMID 16200144.
- ^ Koplow, David A. (2003). Smallpox: the fight to eradicate a global scourge. Berkeley: University of California Press. ISBN 0-520-24220-3.
- ^ "UC Davis Magazine, Summer 2006: Epidemics on the Horizon". Retrieved 2008-01-03.
- ^ How Poxviruses Such As Smallpox Evade The Immune System, ScienceDaily, February 1, 2008
- ^ a b c "Smallpox". WHO Factsheet. Retrieved 2007-09-22.
- ^ Kanda, N (2001). "(Book Review) The Eradication of Smallpox: Edward Jenner and The First and Only Eradication of a Human Infectious Disease". Nature Medicine. 7 (1): 15–6. doi:10.1038/83283. PMID 83283.
{{cite journal}}: More than one of|author=and|last1=specified (help) - ^ Dubochet J, Adrian M, Richter K, Garces J, Wittek R (1994). "Structure of intracellular mature vaccinia virus observed by cryoelectron microscopy". J. Virol. 68 (3): 1935–41. doi:10.1128/jvi.68.3.1935-1941.1994. PMC 236655. PMID 8107253.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ a b Moss B (2006). "Poxviridae: the viruses and their replication". In Fields BN, Knipe DM, Howley PM, et al. (eds) (ed.). Fields Virology. Vol. Vol 2 (5th ed.). Philadelphia, PA: Lippincott-Raven. pp. 2905–46. ISBN 0781760607.
{{cite book}}:|editor=has generic name (help);|volume=has extra text (help)CS1 maint: multiple names: editors list (link) - ^ Damon I (2006). "Poxviruses". In Fields BN, Knipe DM, Howley PM, et al. (eds) (ed.). Fields Virology. Vol. Vol 2 (5th ed.). Philadelphia, PA: Lippincott-Raven. pp. 2947–76. ISBN 0781760607.
{{cite book}}:|editor=has generic name (help);|volume=has extra text (help)CS1 maint: multiple names: editors list (link) - ^ a b c d e f Atkinson W, Hamborsky J, McIntyre L, Wolfe S (eds.) (2005). "Smallpox". Epidemiology and Prevention of Vaccine-Preventable Diseases (The Pink Book) (PDF) (9th ed.). Washington DC: Public Health Foundation. pp. 281–306.
{{cite book}}:|author=has generic name (help)CS1 maint: multiple names: authors list (link) - ^ a b "CDC Smallpox". Smallpox Overview. Retrieved 2007-12-26.
- ^ Henderson DA, Inglesby TV, Bartlett JG; et al. (1999). "Smallpox as a biological weapon: medical and public health management. Working Group on Civilian Biodefense". JAMA. 281 (22): 2127–37. doi:10.1001/jama.281.22.2127. PMID 10367824.
{{cite journal}}: Explicit use of et al. in:|author=(help)CS1 maint: multiple names: authors list (link) - ^ Rao, A. R. (1972). Smallpox. Bombay: Kothari Book Depot. OCLC 723806
- ^ Hogan CJ, Harchelroad F. "CBRNE - Smallpox". eMedicine. Retrieved 2006-09-23.
- ^ "Q Fever: eMedicine Infectious Diseases". Emedicine.medscape.com. 2008-11-18. Retrieved 2010-03-09.
- ^ "Leptospirosis: eMedicine Infectious Diseases". Emedicine.medscape.com. 2008-08-11. Retrieved 2010-03-09.
- ^ a b c d e "Plague: eMedicine Infectious Diseases". Emedicine.medscape.com. Retrieved 2010-03-09.
- ^ "Endocarditis: eMedicine Emergency Medicine". Emedicine.medscape.com. 2009-11-23. Retrieved 2010-03-09.
- ^ Kasper DL, Brunwald E, Fauci AS, Hauser S, Longo DL, Jameson JL (May 2005). Harrison's Principles of Internal Medicine. McGraw-Hill. pp. 731–40. ISBN 0-07-139140-1. OCLC 54501403 56437106 56801936 56967424.
{{cite book}}: Check|oclc=value (help)CS1 maint: date and year (link) CS1 maint: multiple names: authors list (link) - ^ a b c d e f g h i j k l m n o Mitchell RS, Kumar V, Robbins SL, Abbas AK, Fausto N (2007). Robbins Basic Pathology (8th ed.). Saunders/Elsevier. pp. 406–8. ISBN 978-1-4160-2973-1.
{{cite book}}: CS1 maint: multiple names: authors list (link) Cite error: The named reference "Robbins" was defined multiple times with different content (see the help page). - ^ "Pericarditis: Symptoms". MayoClinic.com. Retrieved 2010-03-09.
- ^ "Myocarditis: eMedicine Emergency Medicine". Emedicine.medscape.com. 2009-12-01. Retrieved 2010-03-09.
- ^ "Tuberculosis (Pulmonary Tuberculosis)-Symptoms, How It's Spread, Types". Webmd.com. 2009-04-23. Retrieved 2010-03-09.
- ^ Konstantinos, A (2010). "Testing for tuberculosis". Australian Prescriber, 33:12-18. http://www.australianprescriber.com/magazine/33/1/12/18/
- ^ Jasmer RM, Nahid P, Hopewell PC (December 2002). "Clinical practice. Latent tuberculosis infection" (PDF). N. Engl. J. Med. 347 (23): 1860–6. doi:10.1056/NEJMcp021045. PMID 12466511.
{{cite journal}}: CS1 maint: date and year (link) CS1 maint: multiple names: authors list (link) - ^ a b c Cite error: The named reference
WHO2004datawas invoked but never defined (see the help page). - ^ Cite error: The named reference
WHO2009-Epidemiologywas invoked but never defined (see the help page). - ^ Tuberculosis Symptoms From eMedicineHealth. Author: George Schiffman, MD, FCCP. Last Editorial Review: 1/15/2009
- ^ Additional symptoms for primary/early pulmonary infection: wrongdiagnosis.com --> Diseases » Tuberculosis » Symptoms Retrieved on 1 June 2009
- ^ Extrapulmonary Tuberculosis: An Overview MARJORIE P. GOLDEN, M.D., Yale University School of Medicine and Hospital of Saint Raphael, New Haven, Connecticut. HOLENARASIPUR R. VIKRAM, M.D., Mayo Clinic, Scottsdale, Arizona.
- ^ Centers for Disease Control and Prevention (CDC), Division of Tuberculosis Elimination. Core Curriculum on Tuberculosis: What the Clinician Should Know. 4th edition (2000). Updated August 2003.
- ^ Southwick, Frederick (10 December 2007). "Chapter 4: Pulmonary Infections". Infectious Diseases: A Clinical Short Course, 2nd ed. McGraw-Hill Medical Publishing Division. p. 104. ISBN 978-0071477222.
{{cite book}}: More than one of|pages=and|page=specified (help) - ^ Cox R (2004). "Quantitative relationships for specific growth rates and macromolecular compositions of Mycobacterium tuberculosis, Streptomyces coelicolor A3(2) and Escherichia coli B/r: an integrative theoretical approach". Microbiology. 150 (Pt 5): 1413–26. doi:10.1099/mic.0.26560-0. PMID 15133103.
{{cite journal}}: CS1 maint: unflagged free DOI (link) - ^ a b Madison B (2001). "Application of stains in clinical microbiology". Biotech Histochem. 76 (3): 119–25. doi:10.1080/714028138. PMID 11475314. Cite error: The named reference "Madison_2001" was defined multiple times with different content (see the help page).
- ^ Parish T, Stoker N (1999). "Mycobacteria: bugs and bugbears (two steps forward and one step back)". Mol Biotechnol. 13 (3): 191–200. doi:10.1385/MB:13:3:191. PMID 10934532.
- ^ van Soolingen D, Hoogenboezem T, de Haas PE; et al. (October 1997). "A novel pathogenic taxon of the Mycobacterium tuberculosis complex, Canetti: characterization of an exceptional isolate from Africa". Int. J. Syst. Bacteriol. 47 (4): 1236–45. doi:10.1099/00207713-47-4-1236. PMID 9336935.
{{cite journal}}: Explicit use of et al. in:|author=(help)CS1 maint: date and year (link) CS1 maint: multiple names: authors list (link) - ^ Niemann S, Rüsch-Gerdes S, Joloba ML; et al. (September 2002). "Mycobacterium africanum subtype II is associated with two distinct genotypes and is a major cause of human tuberculosis in Kampala, Uganda". J. Clin. Microbiol. 40 (9): 3398–405. doi:10.1128/JCM.40.9.3398-3405.2002. PMC 130701. PMID 12202584.
{{cite journal}}: Explicit use of et al. in:|author=(help)CS1 maint: date and year (link) CS1 maint: multiple names: authors list (link) - ^ Niobe-Eyangoh SN, Kuaban C, Sorlin P; et al. (June 2003). "Genetic biodiversity of Mycobacterium tuberculosis complex strains from patients with pulmonary tuberculosis in Cameroon". J. Clin. Microbiol. 41 (6): 2547–53. doi:10.1128/JCM.41.6.2547-2553.2003. PMC 156567. PMID 12791879.
{{cite journal}}: Explicit use of et al. in:|author=(help)CS1 maint: date and year (link) CS1 maint: multiple names: authors list (link) - ^ Thoen C, Lobue P, de Kantor I (February 2006). "The importance of Mycobacterium bovis as a zoonosis". Vet. Microbiol. 112 (2–4): 339–45. doi:10.1016/j.vetmic.2005.11.047. PMID 16387455.
{{cite journal}}: CS1 maint: date and year (link) CS1 maint: multiple names: authors list (link) - ^ Pfyffer GE, Auckenthaler R, van Embden JD, van Soolingen D (1998). "Mycobacterium canettii, the smooth variant of M. tuberculosis, isolated from a Swiss patient exposed in Africa". Emerging Infect. Dis. 4 (4): 631–4. doi:10.3201/eid0404.980414. PMC 2640258. PMID 9866740.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ Niemann S, Richter E, Dalügge-Tamm H, Schlesinger H, Graupner D, Königstein B, Gurath G, Greinert U, Rüsch-Gerdes S (2000). "Two cases of Mycobacterium microti derived tuberculosis in HIV-negative immunocompetent patients". Emerg Infect Dis. 6 (5): 539–42. doi:10.3201/eid0605.000516. PMC 2627952. PMID 10998387.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ "Diagnosis and treatment of disease caused by nontuberculous mycobacteria. This official statement of the American Thoracic Society was approved by the Board of Directors, March 1997. Medical Section of the American Lung Association". Am J Respir Crit Care Med. 156 (2 Pt 2): S1–25. 1997. PMID 9279284.
- ^ "Histoplasmosis: Symptoms". MayoClinic.com. Retrieved 2010-03-09.
- ^ "Septic arthritis: MedlinePlus Medical Encyclopedia". Nlm.nih.gov. Retrieved 2010-03-09.
- ^ "Parvovirus infection: Symptoms". MayoClinic.com. Retrieved 2010-03-09.
- ^ "Roseola". Kidshealth.org. Retrieved 2010-03-09.
- ^ "Toxic Shock Syndrome". Emedicinehealth.com. Retrieved 2010-03-09.
- ^ "WHO | Water-related Diseases". Who.int. 2009-02-21. Retrieved 2010-03-09.
- ^ "Hepatitis A: Symptoms". MayoClinic.com. Retrieved 2010-03-09.
- ^ "Cat Scratch Disease". Webmd.com. 2009-04-08. Retrieved 2010-03-09.
- ^ "CDC - RSV: Clinical Description and Diagnosis". Cdc.gov. Retrieved 2010-03-09.
- ^ "Echoviruses: eMedicine Infectious Diseases". Emedicine.medscape.com. 2009-11-25. Retrieved 2010-03-09.
- ^ "Coxsackievirus Infections". Kidshealth.org. Retrieved 2010-03-09.
- ^ "Poliomyelitis (infantile paralysis, polio)". Health.state.ny.us. Retrieved 2010-03-09.
- ^ "Rickettsialpox: eMedicine Infectious Diseases". Emedicine.medscape.com. 2008-04-11. Retrieved 2010-03-09.
- ^ "Trench Fever: eMedicine Infectious Diseases". Emedicine.medscape.com. 2009-02-20. Retrieved 2010-03-09.
- ^ "Human Anaplasmosis Information for Health Professionals - Minnesota Dept. of Health". Health.state.mn.us. 2009-11-30. Retrieved 2010-03-09.
- ^ "Chagas Disease (American Trypanosomiasis): eMedicine Infectious Diseases". Emedicine.medscape.com. 2009-12-17. Retrieved 2010-03-09.
- ^ "Chagas Disease: MedlinePlus". Nlm.nih.gov. Retrieved 2010-03-09.
- ^ "CDC Anthrax Q & A: Signs and Symptoms". Bt.cdc.gov. 2003-06-02. Retrieved 2010-03-09.
- ^ "Legionellosis (Legionnaires' disease)". Health.state.ny.us. Retrieved 2010-03-09.
- ^ "Listeriosis-Topic Overview". Webmd.com. 2009-02-23. Retrieved 2010-03-09.
- ^ "Amebic Meningoencephalitis: eMedicine Pediatrics: General Medicine". Emedicine.medscape.com. 2009-01-21. Retrieved 2010-03-09.
- ^ "WHO | Rift Valley fever". Who.int. 2008-12-29. Retrieved 2010-03-09.
- ^ https://health.google.com/health/ref/Hantavirus>
- ^ "Lymphocytic Choriomeningitis: eMedicine Infectious Diseases". Emedicine.medscape.com. 2009-02-06. Retrieved 2010-03-09.
- ^ "CDC - Division of Foodborne, Bacterial and Mycotic Diseases (DFBMD) - NCZVED". Cdc.gov. Retrieved 2010-03-09.
- ^ "Rotavirus Symptoms, Causes, Treatment, Vaccine and Diagnosis on". Medicinenet.com. Retrieved 2010-03-09.
- ^ "Severe Acute Respiratory Syndrome SARS - Symptoms, Diagnosis, Treatment of Severe Acute Respiratory Syndrome SARS - NY Times Health Information". Health.nytimes.com. 2009-02-03. Retrieved 2010-03-09.
- ^ "Toxoplasmosis: eMedicine Infectious Diseases". Emedicine.medscape.com. 2009-01-27. Retrieved 2010-03-09.
- ^ https://health.google.com/health/ref/Leishmaniasis
- ^ "Staphylococcal Scalded Skin Syndrome: eMedicine Emergency Medicine". Emedicine.medscape.com. 2009-06-18. Retrieved 2010-03-09.
- ^ "Otitis Media: eMedicine Emergency Medicine". Emedicine.medscape.com. Retrieved 2010-03-09.
- ^ https://health.google.com/health/ref/Malignant+otitis+externa
- ^ "Sinusitis: eMedicine Emergency Medicine". Emedicine.medscape.com. Retrieved 2010-03-09.
- ^ "Retropharyngeal Abscess: eMedicine Emergency Medicine". Emedicine.medscape.com. 2009-09-16. Retrieved 2010-03-09.
- ^ "Peritonsillar Abscess: eMedicine Emergency Medicine". Emedicine.medscape.com. 2009-03-11. Retrieved 2010-03-09.
- ^ "Pediatrics, Epiglottitis: eMedicine Emergency Medicine". Emedicine.medscape.com. 2009-01-29. Retrieved 2010-03-09.
- ^ "Rabies: eMedicine Emergency Medicine". Emedicine.medscape.com. 2009-05-11. Retrieved 2010-03-09.
- ^ "Venezuelan Encephalitis: eMedicine Infectious Diseases". Emedicine.medscape.com. 2008-10-10. Retrieved 2010-03-09.
- ^ https://health.google.com/health/ref/Tooth+abscess
- ^ "Brain Abscess: eMedicine Infectious Diseases". Emedicine.medscape.com. 2008-06-26. Retrieved 2010-03-09.
- ^ "Croup: eMedicine Pediatrics: General Medicine". Emedicine.medscape.com. Retrieved 2010-03-09.
- ^ "Kidney infection: Symptoms". MayoClinic.com. Retrieved 2010-03-09.
- ^ "Cystitis: Symptoms". MayoClinic.com. Retrieved 2010-03-09.
- ^ "Cholangitis: eMedicine Emergency Medicine". Emedicine.medscape.com. 2009-09-29. Retrieved 2010-03-09.
- ^ "Pyogenic Hepatic Abscesses: eMedicine General Surgery". Emedicine.medscape.com. 2009-01-23. Retrieved 2010-03-09.
- ^ "Cellulitis, Orbital: eMedicine Ophthalmology". Emedicine.medscape.com. 2009-12-23. Retrieved 2010-03-09.


