User:Gcchemistry/Unbitrium
Category:Chemical elements Category:Hypothetical chemical elements
| Unbitrium | ||||||
|---|---|---|---|---|---|---|
| Pronunciation | /ˌuːnbaɪˈtraɪəm/ | |||||
| Unbitrium in the periodic table | ||||||
| ||||||
| Group | g-block groups (no number) | |||||
| Period | period 8 (theoretical, extended table) | |||||
| Block | g-block | |||||
| Electrons per shell | 2, 8, 18, 32, 35, 18, 8, 2 (predicted) | |||||
| Physical properties | ||||||
| Phase at STP | unknown | |||||
| Atomic properties | ||||||
| Oxidation states | common: (none) | |||||
| Isotopes of unbitrium | ||||||
| Template:infobox unbitrium isotopes does not exist | ||||||
Unbitrium (/uːnˈbaɪtriəm/), (meaning one-two-three-ium[3][4]) also known as eka-protactinium[5][6] or element 123, is the hypothetical chemical element in the periodic table that has the temporary symbol Ubt and has the atomic number 123[7]. According to Leonard Schiff (see section: Electron configuration)[8], unbitrium would be the first element to have G electrons (Schiff predicts that unbiunium and unbibium will have only S electrons and D electrons[9].). Unbitrium is expected to be on an island of stability.[10]
History
[edit]The name unbitrium is used as a placeholder, such as in scientific articles about the search for element 123. Transuranic elements beyond californium are always artificially produced, and usually end up being named for a scientist or the location of a laboratory that does work in atomic physics.
Naming
[edit]The name unbitrium is a systematic element name, used as a placeholder until it is confirmed by other research groups and the IUPAC decides on a name. Usually, the name suggested by the discoverer(s) is chosen.[4][11][12][13]
Eka-protactinium
[edit]Unbitrium is also known as eka-protactinium, but unquadunium or unquadtrium could also be known as eka-protactinium[14][5].
See Mendeleev's predicted elements.
Other names for unbitrium
[edit]- Element 123
- Dvi-praseodymium
Position in the periodic table
[edit]Unbitrium is expected to be in a new group of elements called superactinides[15]. These should behave differently from other groups of elements (those already known).
Isotopes
[edit]Calculations have shown that 326Ubt would be the most stable isotope[16][13]. The closed neutron shells say that 307Ubt and 319Ubt would be the most stable isotopes.[10]
Synthesis
[edit]As of December 2012, no attempt has ever been made to synthesize element 123. Right now, there aren't any planned attempts to synthesize unbitrium either[n 1][17].
Stable unbitrium
[edit]The only practical isotopes that would be longer-lived than others are 307Ubt (N=184) and 319Ubt (N=196)[10]. They would be relatively stable. However, unbitrium is not expected to be the most stable element in the island of stability. Unbihexium is expected to be the most stable element on the island of stability.[18]
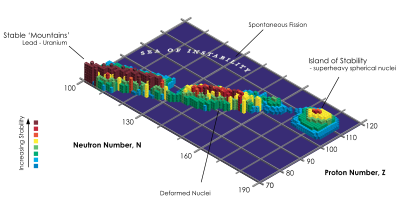
The stability of nuclei decreases greatly with the increase in atomic number after plutonium, the heaviest primordial element, so that all isotopes with an atomic number above 101 decay radioactively with a half-life under a day, with an exception of dubnium-268. No elements with atomic numbers above 82 (after lead) have stable isotopes.[19] Nevertheless, because of reasons not very well understood yet, there is a slight increased nuclear stability around atomic numbers 110–114, which leads to the appearance of what is known in nuclear physics as the "island of stability". This concept, proposed by University of California professor Glenn Seaborg, explains why superheavy elements last longer than predicted.[20] In this region of the periodic table, N=184 and N=196 have been suggested as closed neutron shells. Therefore the isotopes of most interest are 307Ubt and 319Ubt, for these might be considerably longer-lived than other isotopes. Element 123 is predicted to lie within the island of stability, with element 126 predicted to lie near its peak.
Electron configuration
[edit]Leonard I. Schiff[8] predicted that unbitrium would have the electron configuration [Uuo] 8s2 5g2 7d1. That means that unbitrium would be the first element to have g electrons.[9]
Predicted chemical properties
[edit]Unbitrium would probably be a solid under standard conditions (if a large enough quantity is ever made).[21]
Fictional Reference to Unbitrium (Element 123)
[edit]In the fictional universe of Star Trek: The Next Generation, this element was apparently discovered and named. The episode "Rascals" depicted a "trans-periodic table," in a schoolroom set, which depicted element number 123 as being named jamesium, symbol Rj, having an atomic weight of 326[6]. The graphic was created by set artists, probably a homage to designer Richard D. James, and listed this element in the fictional "gamma series."[4][11][12][22]
References
[edit]- ^ http://www.chemeurope.com/en/encyclopedia/Unbitrium.html
- ^ http://www.chemicalaid.com/reference/future_elements.php
- ^ See Systematic element name
- ^ a b c http://biosphere.biologydaily.com/biology/Unbitrium
- ^ a b http://www.wordnik.com/words/unbitrium
- ^ a b http://state.chemistrydaily.com/chemistry/Unbitrium
- ^ http://en.wiktionary.org/wiki/unbitrium
- ^ a b Leonard I. Schiff; Quantum Mechanics, third edition, p. 428, McGraw-Hill, Inc., New York, 1968.
- ^ a b See period 8 element
- ^ a b c See Island of stability
- ^ a b Foundation, Wikimedia. "Extensions od the Periodic Table".
{{cite journal}}: Cite journal requires|journal=(help) - ^ a b http://www.chemeurope.com/en/encyclopedia/Unbitrium.html
- ^ a b http://nicedefinition.com/Definition/Word/unbitrium/unbitrium.aspx
- ^ See extended periodic table
- ^ see superactinide
- ^ http://www.princess-it.com/kp9/hrh-projects/file/20060327_sammakkee/lanchang/element/elements/123.htm
- ^ http://www.thefullwiki.org/Unbitrium
- ^ See unbihexium
- ^ Marcillac, Pierre de (2003). "Experimental detection of α-particles from the radioactive decay of natural bismuth". Nature. 422 (6934): 876–878. Bibcode:2003Natur.422..876D. doi:10.1038/nature01541. PMID 12712201.
{{cite journal}}: Unknown parameter|coauthors=ignored (|author=suggested) (help); Unknown parameter|month=ignored (help) - ^ Kulik, Glenn D. (2002). Van Nostrand's scientific encyclopedia (9 ed.). Wiley-Interscience. ISBN 978-0-471-33230-5. OCLC 223349096.
{{cite book}}:|first2=missing|last2=(help); More than one of|last1=and|last=specified (help) - ^ http://en.wikidoc.org/index.php/Unbitrium
- ^ Emsley, John (2011). Nature's Building Blocks: An A-Z Guide to the Elements (New ed.). New York, NY: Oxford University Press. p. 589. ISBN 978-0-19-960563-7.
Notes
[edit]- ^ This section will be updated if there are any attempts or planned attempts to synthesize unbitrium
External links
[edit]See also
[edit]Category:Chemical elements Category:Nuclear physics Category:Truss elements
