User:Floweryard/sandbox
 | This is a user sandbox of Floweryard. You can use it for testing or practicing edits. This is not the sandbox where you should draft your assigned article for a dashboard.wikiedu.org course. To find the right sandbox for your assignment, visit your Dashboard course page and follow the Sandbox Draft link for your assigned article in the My Articles section. |
Hot start PCR is a modified form of conventional polymerase chain reaction (PCR) that reduces the presence of undesired products and primer dimers due to non-specific DNA amplification at room (or colder) temperatures[1][2]. Non-specific binding is minimized by completing the reaction mix after denaturation[3]. Some ways to complete reaction mixes at high temperatures involve modifications that block DNA polymerase activity in low temperatures[1][4], use of modified deoxyribonucleotide triphosphates (dNTPs)[5], and the physical addition of one of the essential reagents after denaturation[6].
Background
[edit]Polymerase chain reaction (PCR) is a molecular biology technique used to amplify specific DNA segments by several orders of magnitude. It uses DNA polymerase, which is slightly active at low temperatures[1]. In conventional PCR, the reaction mix is completed at room temperature, and due to DNA polymerase activity, primers may form primer dimers or anneal to DNA non-specifically. During the PCR procedure, DNA polymerase will extend any piece of DNA with bound primers, generating target products but also nonspecific products which lower the yield. In hot start PCR, some of the reagents are kept separate until the mixture is heated to the specific annealing temperature. This reduces annealing time, which in turn reduces the likelihood of non-specific DNA extension and the influence of non-specific primer binding prior to denaturation[7][3].
Hot start PCR is especially useful in place of conventional PCR if the reaction mix contains little DNA[8], if there is expected non-specific binding in a multiplex PCR reaction[7], and if the expected amplicon sizes are very small (less than 150bp)[7].
Methods
[edit]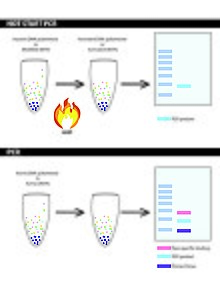
The general principle of hot start PCR is the isolation of one or more reagents from the reaction mix, until the mixture is heated up to denaturation temperature[6].
Physical Separation
[edit]Early methods of hot start PCR involved excluding or limiting the concentration of one of the reagents, until the denaturation stage begins[6]. Over time, a wax-isolation technique was developed whereby a wax layer isolated the reagent until the initial stage of PCR, when it dissolved and released the isolated reagent[9][10].
DNA polymerase inactivation
[edit]DNA polymerase inactivation is a method where the DNA polymerase is kept inactive until the PCR mix is prepared. This can be done using antibodies, and aptamers (chemical antibodies), affibodies (smaller antibody-mimics targetting peptides), as well as chemical modifications[4]. Antibody/affibody/aptamer inactivation of DNA polymerase is more suited for situations requiring a fast inactivation time. When the inactivation of DNA polymerase is not time-sensitive, chemical modifications can be used[4].
Deoxyribonucleotide triphosphate (dNTP) modifications
[edit]Chemically modified deoxyribonucleotide triphosphates (dNTPs[5]) start binding to the template DNA only after the reaction mix is heated up[11].
Advantages
[edit]Hot start PCR significantly reduces nonspecific priming, formation of primer-dimers, and often increases product yields[7]. It also requires less handling and reduces the risk of contamination[12].
Disadvantages
[edit]If the DNA polymerase is chemically modified, the re-activation time during the initial denaturation stage of the PCR cycle is increased because there is a waiting period while the enzyme activates. This increased heating time can damage DNA. Additionally, studies have shown that some chemical modifications can cause issues in amplifying long strands of DNA[4].
References
[edit]- ^ a b c Sharkey, David J.; Scalice, Edward R.; Christy, Kenneth G.; Atwood, Susan M.; Daiss, John L. (1994-05-14). "Antibodies as Thermolabile Switches: High Temperature Triggering for the Polymerase Chain Reaction". Bio/Technology. 12 (5): 506–509. doi:10.1038/nbt0594-506. ISSN 0733-222X.
- ^ Paul, Natasha; Shum, Jonathan; Le, Tony (2010), "Hot Start PCR", Methods in Molecular Biology, Humana Press, pp. 301–318, ISBN 9781607616283, retrieved 2019-10-03
- ^ a b van Pelt-Verkuil, Elizabeth; van Belkum, Alex; Hays, John P., eds. (2008), "Variants and Adaptations of the Standard PCR Protocol", Principles and Technical Aspects of PCR Amplification, Springer Netherlands, pp. 231–276, doi:10.1007/978-1-4020-6241-4_12, ISBN 9781402062414, retrieved 2019-10-03
- ^ a b c d "How is Hot-Start Technology Beneficial For Your PCR - US". www.thermofisher.com. Retrieved 2019-10-03.
- ^ a b "DNTP - The School of Biomedical Sciences Wiki". teaching.ncl.ac.uk. Retrieved 2019-10-09.
- ^ a b c Diagnostic molecular pathology. Coleman, William B. [London]: Elsevier Academic Press. 2016. ISBN 9780128011577. OCLC 960448665.
{{cite book}}: CS1 maint: others (link) - ^ a b c d Birch, David E.; Kolmodin, L.; Wong, J.; Zangenberg, G. A.; Zoccoli, M. A.; McKinney, N.; Young, K. K. Y. (1996-05-30). "Simplified hot start PCR". Nature. 381 (6581): 445–446. doi:10.1038/381445a0. ISSN 1476-4687. Retrieved 2019-10-01.
- ^ Li, H.; Cui, X.; Arnheim, N. (1990-03-01). "Direct electrophoretic detection of the allelic state of single DNA molecules in human sperm by using the polymerase chain reaction". Proceedings of the National Academy of Sciences of the United States of America. 87 (12): 4580–4584. doi:10.1073/pnas.87.12.4580. ISSN 0027-8424. PMID 1972276.
- ^ Kaijalainen, S.; Karhunen, P.J.; Lalu, K.; Lindström, K. (1993). "An alternative hot start technique for PCR in small volumes using beads of wax-embedded reaction components dried in trehalose". Nucleic Acids Research. 21 (12): 2959–2960. doi:10.1093/nar/21.12.2959. ISSN 0305-1048.
- ^ Blair, P.; Ramanujam, R.; Burdick, B. A. (1994-12-01). "Wax-embedded PCR reagents". Genome Research. 4 (3): 191–194. ISSN 1088-9051. PMID 7580906.
- ^ "dNTP Mediated Hot Start PCR Protocol". Sigma-Aldrich. Retrieved 2019-10-09.
- ^ Primrose, S. B. (2001). Principles of gene manipulation. Twyman, Richard M., Old, R. W. (6th ed ed.). Oxford: Blackwell Scientific. ISBN 0632059540. OCLC 48976682.
{{cite book}}:|edition=has extra text (help)
