User:Bigstaceysnider/Condensed aerosol fire suppression

Condensed aerosol fire suppression is a particle-based form of fire extinction similar to gaseous fire suppression or dry chemical fire extinction. As with gaseous fire suppressants, condensed aerosol suppressants use clean agents to suppress the fire. The agent can be delivered by means of mechanical operation, electric operation, or combined electro-mechanical operation. To the difference of gaseous suppressants, which emit only gas, and dry chemical extinguishers, which release powder-like particles of a large size (25-150 microns) condensed aerosols are defined by the National Fire Protection Association as releasing finely-divided solid particles (generally <10 microns), usually in addition to gas.[1]
Whereas dry chemical systems must be directly aimed at the flame, condensed aerosols are flooding agents and therefore effective regardless of the location and height of the fire. Wet chemical systems, such as the kind generally found in foam extinguishers, must, similarly to dry chemical systems, be sprayed directionally, onto the fire. Additionally, wet chemicals (such as potassium carbonate) are dissolved in water, whereas the agents used in condensed aerosols are microscopic solids.
Means of Fire Extinction
[edit]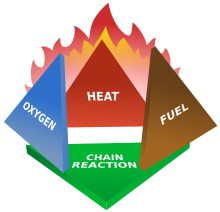
Condensed aerosol suppressants, like gaseous suppressants, use four methods to extinguish fires. They act on the four elements of the "fire tetrahedron," the disparate components that combine to create the chemical reaction underlying any fire. These four means of fire extinction are:
1. Reduction or isolation of fuel 1.5-ثفغثقفغغقثفغغفثغف
2. Reduction of heat
3. Reduction or isolation of oxygen
4. Inhibiting the chain reaction of the above components
Condensed aerosols’ primary operating mechanism follows the third and fourth methods, of inhibiting the fire’s combustion and of hampering the chain reaction of the fire’s various components. By dispersing solid aerosol particulates (usually potassium nitrate, which, when burned, produces potassium carbonate (K2CO3)) that interact with the free radicals of the flame. Once these particles are assimilated into the air feeding oxygen to the base of the flame, they absorb the fire’s heat. The potassium radicals (K) bond with the hydroxide (OH), hydrogen (H) and oxygen (O) radicals which form the flame, producing harmless by-product molecules such as potassium hydroxide (KOH) and water (H2O). Disrupting the reactions necessary to sustain the flame's combustion, the aerosol particulates terminate the fire.
The simplified termination reactions are:
K. + OH = KOH KOH + H = K + H2O
On top of the primary mechanism that inhibits the fire’s combustion reaction, condensed aerosols also operate on the basis of the second fire tetrahedron extinction method: heat reduction. The ultra-fine cooling particles discharged by the condensed aerosol device cool the flame by engulfing it. Though the surface area of each particle is extremely small, the large quantity of particles surrounding the flame offers a sufficiently large combined surface area to absorb a significant portion of the flame’s heat.
Attacking most elements of the fire tetrahedron, condensed aerosol fire suppression agents are among the more effective flame-extinguishing agents. For example, some condensed aerosol fire suppressants can extinguish a Class B flammable liquid pool fire with 1/5th the amount of Halon 1301 agent or 1/10th the amount of a hydrofluorocarbon or fluoroketone based clean agent gaseous fire suppression system in terms of kilogram mass of agent per cubic meter.[2]
Performance
[edit]The extinguishing performance of condensed aerosol fire suppressants is dependent on the density of aerosol particulates in the immediate vicinity of the flame. As with gaseous fire suppression systems, the faster the agent can build around the flame, the more efficient the extinguishing agent will be in terminating the flame’s combustion process. The extinguishing and design densities of aerosol fire suppression agents are generally expressed in kilograms per cubic meter (kg/m^3). Thus, the efficiency of aerosol extinguishing agents varies depending on a number of factors, such as the location of the aerosol relative to the flame, the proximity of other combustible flammable materials, the type of fuel involved, etc.
Condensed aerosol devices are designed to provide a controlled discharge. The aerosol-forming compound is installed inside of the device, which is then fitted with an electric or mechanical initiator. The electric initiator is interfaced with a fire detection control unit or panel, which can be remotely operated by physical means such as by cable, hand operated with a fuse mechanism such as those used in smoke dispensing grenades, or automatic and self-triggering when outfitted with an integral heat-sensing device.
Uses and Applications
[edit]There are two uses for applying fire extinguishing agents: as a total flooding fire protection system or as a local application fire suppression system.
To provide total flooding fire suppression, the total quantity of aerosol required to extinguish a fire inside of fixed space must be determined. The corresponding number of aerosol devices that would collectively discharge the aerosol quantity required are then mounted, typically on the ceiling or wall. Aerosol devices equipped with electric initiators are interconnected and relayed by a fire alarm control panel. Because the aerosol devices are self-contained and function as both a storage container and as a nozzle that propels the gas, no distribution network is required to transport or distribute the fire-extinguishing agent from a remote storage location, resulting in floor space savings and transportation efficiency gains.
Local application fire suppression is typically applied by a handheld portable device tossed directly toward the fire. Unlike streaming portable fire extinguishing units, the operators are not required to place themselves at risk by approaching the fire while applying the extinguishing agent directly at the flames. The portable condensed aerosol device is typically designed to disperse aerosol in a 360° spray pattern, forming a large aerosol cloud around the vicinity of the fire. The aerosol immediately attacks the flames as its particles approach the fire and generate flame-neutralizing potassium radicals. The flames are suppressed as long as the aerosol retains sufficient density. If the aerosol fails to achieve sufficient density to extinguish the fire, it will still suppress the fire, which will retain significantly lower heat. This offers firefighters, for instance, a tool to bring down flames to a manageable heat level and reduce room temperatures while the hose team enters the burning area. As another example, First Responders can deploy condensed aerosols within an enclosed area to suppress fires while evacuating occupants to safety.[3]
Condensed aerosol systems are suitable for special hazards applications as replacements for Halon 1301 systems and high-pressure carbon dioxide systems. Aerosol systems can also be used as alternatives or complements for clean agent gaseous suppressants or water-mist systems.[4]
Environmental Issues
[edit]The United States Environmental Protection Agency has approved condensed aerosol fire suppression systems as acceptable substitutes for Halon 1301 in Total Flooding Systems.[5]. Aerosol extinguishers are also non-ozone depleting and carry little or no global warming potential.[citation needed]
See Also
[edit]- Automatic fire suppression
- Gaseous fire suppression
- Fast flow extinguisher
- Fire extinguisher
- Fire protection engineering
Further Reading
[edit]Notes
[edit]References
[edit]- ^ National Fire Protection Association, "Report on Aerosol Extinguishing Technology,".
- ^ Building and Fire Research Laboratory, National Institute for Standards and Technology, "Encapsulated Micron Aerosol Agents,".
- ^ [1]
- ^ Building and Fire Research Laboratory, National Institute for Standards and Technology, "Encapsulated Micron Aerosol Agents,".
- ^ U.S. Environmental Protection Agency, "Substitutes for Halon 1301 as a Total Flooding Agent,".
External links
[edit]
