User:Benzene039/Aldonic acid
| This is the sandbox page where you will draft your initial Wikipedia contribution.
If you're starting a new article, you can develop it here until it's ready to go live. If you're working on improvements to an existing article, copy only one section at a time of the article to this sandbox to work on, and be sure to use an edit summary linking to the article you copied from. Do not copy over the entire article. You can find additional instructions here. Remember to save your work regularly using the "Publish page" button. (It just means 'save'; it will still be in the sandbox.) You can add bold formatting to your additions to differentiate them from existing content. |
Aldonic Acids
[edit]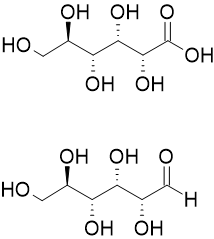
From Wikipedia, the free encyclopedia
Aldonic acids are sugar acids with the general chemical formula, HO2C(CHOH)nCH2OH. They are obtained by oxidizing the aldehyde (-CHO group) of an aldose to form a carboxylic acid (-COOH group).[1] Aldonic acids are generally found in their ring form. However, these rings do not have a chiral carbon at the terminal end bearing the aldehyde, and they cannot form R−O−R′ linkages between different molecules.[2]
The nomenclature of aldonic acids and their lactones is based on replacing the suffix "-ose" with "onic acid" or "onolactone". Hence, D-glucose is oxidized to D-gluconic acid and D-gluconolactone.[3]
Synthesis
[edit]Oxidation by Bromine and Water
[edit]Aldonic acids are most commonly prepared by the oxidation of the sugar with bromine and water under neutral pH.[4]

Strecker Reaction
Alternatively, they arise by homologation of an aldose using the Strecker reaction.[5] Cyanide in ammonia reacts with an aldose to produce an intermediate, which is then reacted with a hydronium ion to form an aldonic acid.
Oxidation by Benedict's and Fehling's Reagents
[edit]Aldonic acids are the products of the oxidation of aldoses by Benedict's or Fehling's reagents.[6] Copper ions react with an aldose to form a red precipitate, Cu2O.

Natural Synthesis
Anaerobic bacteria can also perform dehydrogenation to produce aldonic acids.[7] This is done by synthesizing enzymes that are able to selectively oxidize aldoses to their corresponding aldonic acid.
Applications
[edit]In commercial settings, glucose, galactose, or arabinose are commonly oxidized to obtain aldonic acids.[7] These products can then be used as the building blocks for preservatives, buffering agents, and other chemicals.[7] As such, the use of aldonic acids for chemical applications is of growing interest to various industries.
Aldonic acids can be used as the natural starting materials to synthetic products[8] including polyesters and polyurethane.[9] The incorporation of these organic sugars into synthetic materials allow for a more renewable alternative to oil-based polymer synthesis,[9] and increased structural durability within polymer chains.[10]
Properties
[edit]Aldonic acids are typically used in industrial applications for their ability to degrade naturally in the environment.[9] This can be attributed to their affinity with water, as the polar bonds within the carboxylic acid group of aldonic acids allow them to interact with aquatic systems.[11]
The structural diversity of aldonic acids also allow for various properties. Their ring formation creates an added layer of rigidity when integrated with other materials.[10]
References
[edit]- ^ Gold, Victor, ed. (2019). The IUPAC Compendium of Chemical Terminology: The Gold Book (4 ed.). Research Triangle Park, NC: International Union of Pure and Applied Chemistry (IUPAC). doi:10.1351/goldbook.a00212.
- ^ Sartori, Suélen Karine; Diaz, Marisa Alves Nogueira; Diaz-Muñoz, Gaspar (2021-03-26). "Lactones: Classification, synthesis, biological activities, and industrial applications". Tetrahedron. 84: 132001. doi:10.1016/j.tet.2021.132001. ISSN 0040-4020.
- ^ Robyt, John F. (1998). Essentials of carbohydrate chemistry. Springer advanced texts in chemistry. New York: Springer. ISBN 978-0-387-94951-2.
- ^ Isbell, Horace S. (March 1962). "Oxidation of aldoses with bromine". Journal of Research of the National Bureau of Standards Section A: Physics and Chemistry. 66A (3): 233. doi:10.6028/jres.066a.023. ISSN 0022-4332. PMC 5310681.
{{cite journal}}: CS1 maint: PMC format (link) - ^ "d-gulonic-y-lactone". Organic Syntheses. 36: 38. 1956. doi:10.15227/orgsyn.036.0038. ISSN 0078-6209.
- ^ Simoni, Robert D.; Hill, Robert L.; Vaughan, Martha (April 2002). "Benedict's Solution, a Reagent for Measuring Reducing Sugars: the Clinical Chemistry of Stanley R. Benedict". Journal of Biological Chemistry. 277 (16): e5–e6. doi:10.1016/s0021-9258(19)61050-1. ISSN 0021-9258.
- ^ a b c Wieschalka, Stefan; Blombach, Bastian; Bott, Michael; Eikmanns, Bernhard J. (March 2013). "Bio‐based production of organic acids with C orynebacterium glutamicum". Microbial Biotechnology. 6 (2): 87–102. doi:10.1111/1751-7915.12013. ISSN 1751-7915. PMC 3917452. PMID 23199277.
{{cite journal}}: CS1 maint: PMC format (link) - ^ Mehtiö, Tuomas; Toivari, Mervi; Wiebe, Marilyn G.; Harlin, Ali; Penttilä, Merja; Koivula, Anu (2016-09-02). "Production and applications of carbohydrate-derived sugar acids as generic biobased chemicals". Critical Reviews in Biotechnology. 36 (5): 904–916. doi:10.3109/07388551.2015.1060189. ISSN 0738-8551.
- ^ a b c Galbis, Juan A.; García-Martín, M. de Gracia; de Paz, M. Violante; Galbis, Elsa (2016-02-10). "Synthetic Polymers from Sugar-Based Monomers". Chemical Reviews. 116 (3): 1600–1636. doi:10.1021/acs.chemrev.5b00242. ISSN 0009-2665.
- ^ a b Gregory, Georgina L.; López-Vidal, Eva M.; Buchard, Antoine (2017-02-14). "Polymers from sugars: cyclic monomer synthesis, ring-opening polymerisation, material properties and applications". Chemical Communications. 53 (14): 2198–2217. doi:10.1039/C6CC09578J. ISSN 1364-548X.
- ^ Morzyk-Ociepa, Barbara; Michalska, Danuta; Pietraszko, Adam (January 2004). "Structures and vibrational spectra of indole carboxylic acids. Part I. Indole-2-carboxylic acid". Journal of Molecular Structure. 688 (1–3): 79–86. doi:10.1016/j.molstruc.2003.09.027. ISSN 0022-2860.
