User:AshleyHardy/Congenital distal spinal muscular atrophy
Congenital distal spinal muscular atrophy (congenital dSMA) is a hereditary genetic condition characterized by muscle wasting (atrophy), particularly of distal muscles in legs and hands, and by early - onset contractures of the hip, knee, and ankle. Affected individuals often have shorter lower limbs relative to the trunk and upper limbs. The condition is a result of a loss of anterior horn cells localized to lumbar and cervical regions of the spinal cord early in infancy, which in turn is caused by a mutation of the TRPV4 gene. The disorder is inherited in an autosomal dominant manner.[1] Arm muscle and function, as well as cardiac and respiratory functions are typically well preserved.[2]
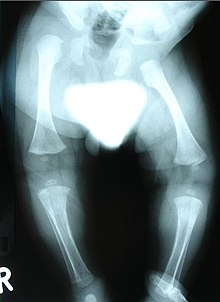
Signs & Symptoms
[edit]- Neurogenic muscle weakness
- Atrophy (of lower and upper limbs)
- Club_foot
- Arthrogryposis
- Scoliosis
- Platyspondyly
- Pes cavus
- Vocal cord paralysis
Causes
[edit]
Congenital distal spinal muscular atrophy is caused by a mutation of the TRPV4 gene found on the 12q23-q24.1.[3] The mutation causes an affected individual to have lower levels of TRPV4 expression. This deficiency can lead to abnormal osmotic regulation. Congenital dSMA is genetically heterogeneous, meaning a mutation on this gene can cause a plethora of other phenotypically related or phenotypically unrelated diseases depending on the region that is mutated.
TRPV4
[edit]The TRPV4 (transient receptor potential vanilloid 4) gene, located on chromosome 12, encodes for a protein that serves as an ion channel, typically found in the plasma membrane and is permeable to Ca2+. TRPV4 plays a major role in mechanosensation, as well as osmosensory functions in endothelia and alveoli.[4] The TRPV4 protein consists of 871 amino acids with it's N- and C- terminals facing intracellularly. The protein is also comprised of six alpha helices that pass through the plasma membrane. [3]
Ankyrin Repeat Domain (ARD)
[edit]The ARD is located near the intracellular N-terminal of the TRPV4 protein and consists of six ankyrin repeats. Four missense mutations have been identified at three specific positions all located within the ARD. All of these mutations are due to the substitution of arginine residues.[5]
Identified Mutations
[edit]- R296H, arginine to histidine substitution
- R315W, arginine to tryptophan substitution
- R316C, arginine to cysteine substitution
- R594H, arginine to histidine substitution
Testing
[edit]
- Nerve conduction studies (NCS), to test for denervation
- Electromyography (EMG), also to detect denervation
- X-ray, to look for bone abnormalities
- Magnetic Resonance Imaging (MRI)
- Skeletel muscle biopsy examination
- Serum creatine kinase (CK) level in blood, usually elevated in affected individuals
- Pulmonary function test
Diagnosis
[edit]A diagnosis is made after specific criteria have been satisfied. Electrophysiological evidence of denervation with intact motor and sensory nerve conduction findings must be made by using NCSs, usually in conjunction with EMG. An x-ray of an individual will also show abnormal bone growth Histologic evidence from muscle biopsy samples of denervation must also be present.[6]
Management
[edit]Congenital dSMA has a relatively stable disease course, with disability mainly attributed to increased contractures rather than loss of muscle strength. Individuals frequently use crutches, knee ankle foot orthoses, callipers, or wheelchairs.[2] Orthopaedic surgery can be an option for some patients. Physical therapy and occupational therapy can help prevent further contractures from occuring, though they do not reverse the effects of preexisting ones.[7]
Related Disorders Involving the TRPV4 Gene
[edit]Mutations on the TRPV4 gene can cause different, but very similar spinal muscular atrophies (SMA) of the peripheral nervous system. Other skeletal dysplasias can also be the result of a mutation on the TRPV4 gene.[8] The mutations can occur at different alleles that have been linked to scapuloperoneal spinal muscular atrophy (SPSMA) and hereditary motor and sensory neuropathy 2C (HMSN2C), which are forms of SMAs. The related skeletal dysplasias include brachyolmia, spondylometaphyseal dysplasia Kozlowski type, and nonlethal metatropic dysplasia.[9] All of the disorders exhibit overlapping symptoms, as well as unique ones that set them apart from the others.
Scapuloperoneal Spinal Muscular Atrophy (SPSMA)
[edit]Scapuloperoneal weakness and atrophy, vocal cord paralysis, laryngeal palsy, along with absence of some muscle groups are all typical features of SPSMA. Males are more affected than females, and later generations have increased disease expression, suggesting genetic anticipation.[10]
Hereditary Motor and Sensory Neuropathy 2C (HMSN2C)
[edit]Hereditary motor and sensory neuropathy 2C, or commonly known as Charcot - Marie - Tooth disease is also a result of a mutation of the TRPV4 gene. Signs and symptoms include distal muscle weakness and wasting in the limbs, distal sensory loss, vocal cord paralysis, and weakness of the diaphragm and intercostal muscles. Onset varies greatly from childhood to 30 years of age, with prognosis mainly due to respiratory complications.[11]
Brachyolmia
[edit]Features of brachyolmia include short trunk, scoliosis, and mild short stature. The autosomal dominant form of brachyolmia is also linked to a mutation on the TRPV4 gene located on chromosome 12q24.1-12q24.2.[12]
Spondylometaphyseal dysplasia Kozlowski type (SMDK)
[edit]Affected individuals typically have short trunk and short stature, in addition to bowlegs and a waddling gait. Kyphoscoliosis is common and the thorax is generally short and broad. Further features include severe platyspondyly, and metaphyseal dysplasia.[13]
Nonlethal metatropic dysplasia (MD)
[edit]The course of the disease is very unique in that it evolves from individuals being short-limbed at birth to instead possessing a short-trunk phenotype over the first decade, as a result of rapidly progressing kyphoscoliosis. At birth, a prominent forehead and squared jaw are present. Occasionally, an individual may be born with an elongated coccyx, sometimes referred to as a tail-like appendage. [13]
See also
[edit]References
[edit]- ^ Oates, E.C. (2012). "Autosomal dominant congenital spinal muscular atrophy: a true form of spinal muscular atrophy caused by early loss of anterior horn cells". Brain: A Journal of Neurology. 135 (6): 1714–1723. doi:10.1093/brain/aws108. PMID 22628388.
{{cite journal}}: Unknown parameter|coauthors=ignored (|author=suggested) (help) - ^ a b Mercuri, E. (2004). "Congenital form of spinal muscular atrophy predominantly affecting the lower limbs: a clinical and muscle MRI study". Neuromuscular Disorders. 14 (2): 125–129. doi:10.1016/j.nmd.2003.09.005. PMID 14733958.
{{cite journal}}: Unknown parameter|coauthors=ignored (|author=suggested) (help) - ^ a b Everaerts, W. (2010). "The vanilloid transient receptor potential channel TRPV4: from structure to disease". Progress in Biophysics and Molecular Biology. 103 (1): 2–17. doi:10.1016/j.pbiomolbio.2009.10.002. PMID 19835908.
{{cite journal}}: Unknown parameter|coauthors=ignored (|author=suggested) (help) - ^ Auer-Grumbach, M. (2010). "Alterations in the ankyrin domain of TRPV4 cause congenital distal SMA, scapuloperoneal SMA and HMSN2C". Nature Genetics. 42 (2): 160–164. doi:10.1038/ng.508. PMC 3272392. PMID 20037588.
{{cite journal}}: Unknown parameter|coauthors=ignored (|author=suggested) (help) - ^ Dai, J. (2010). "TRPV4-pathy, a novel channelopathy affecting diverse systems". Journal of Human Genetics. 55 (7): 400–402. doi:10.1038/jhg.2010.37. PMID 20505684.
{{cite journal}}: Unknown parameter|coauthors=ignored (|author=suggested) (help) - ^ Russman, B.S. (2007). "Spinal muscular atrophy: clinical classification and disease heterogeneity". Journal of Child Neurology. 22 (8): 946–951. doi:10.1177/0883073807305673. PMID 17761648.
- ^ Farmer, S.E. (2001). "Contractures in orthopaedic and neurological conditions: a review of causes and treatment". Disability and Rehabilitation. 23 (13): 549–558. doi:10.1080/09638280010029930. PMID 11451189.
{{cite journal}}: Unknown parameter|coauthors=ignored (|author=suggested) (help) - ^ Krakow, D. (2009). "Mutations in the gene encoding the calcium-permeable ion channel TRPV4 produce spondylometaphyseal dysplasia, Kozlowski type and metatropic dysplasia". American Journal of Human Genetics. 84 (3): 307–315. doi:10.1016/j.ajhg.2009.01.021. PMC 2667978. PMID 19232556.
{{cite journal}}: Unknown parameter|coauthors=ignored (|author=suggested) (help) - ^ Camacho, N. (2010). "Dominant TRPV4 mutations in nonlethal and lethal metatropic dysplasia". American Journal of Medical Genetics. 152A (5): 1169–1177. doi:10.1002/ajmg.a.33392. PMC 4169191. PMID 20425821.
{{cite journal}}: Unknown parameter|coauthors=ignored (|author=suggested) (help) - ^ Isozumi, K. (1996). "Linkage of scapuloperoneal spinal muscular atrophy to chromosome 12q24.1-12q24.31". Human Molecular Genetics. 5 (9): 1377–1382. doi:10.1093/hmg/5.9.1377. PMID 8872481.
{{cite journal}}: Unknown parameter|coauthors=ignored (|author=suggested) (help) - ^ Zimoń, M. (2010). "Dominant mutations in the cation channel gene transient receptor potential vanilloid 4 cause an unusual spectrum of neuropathies". Brain: A Journal of Neurology. 133 (Pt 6): 1788–1809. doi:10.1093/brain/awq109. PMC 2912694. PMID 20460441.
{{cite journal}}: Unknown parameter|coauthors=ignored (|author=suggested) (help) - ^ Rock, M.J. (2008). "Gain-of-function mutations in TRPV4 cause autosomal dominant brachyolmia". Nature Genetics. 40 (8): 999–1003. doi:10.1038/ng.166. PMC 3525077. PMID 18587396.
{{cite journal}}: Unknown parameter|coauthors=ignored (|author=suggested) (help) - ^ a b Nishimura, G. (2012). "TRPV4-associated skeletal dysplasias". American Journal of Medical Genetics. 160C (3): 190–204.
{{cite journal}}: Unknown parameter|coauthors=ignored (|author=suggested) (help)
External Links
[edit]Online Mendelian Inheritance in Man (OMIM)
Connective Tissue Gene Tests (CTGT)
