User:Anthonyhcole/Parkinson's disease
Parkinson's disease (PD, also known as idiopathic parkinsonism) is a degenerative disorder of the central nervous system mainly affecting the motor system. Many of the motor symptoms of PD result from the loss of pigmented dopamine-generating cells in the substantia nigra, a region of the midbrain. The causes of this cell death are poorly understood. A progressive reduction in the speed and amplitude of voluntary movement is the only physical sign present in all patients and is responsible for the common early complaints of loss of dexterity, writing difficulties, clumsiness and difficulty walking. Muscular stiffness of the limbs and trunk are also common. Trembling or tremor of one limb at rest, although not necessarily present in all cases, is the commonest symptom leading to accurate diagnosis. Dysfunction of the autonomic nervous system is also common leading to constipation, swallowing and bladder problems and disturbed temperature regulation with excessive sweating. Depression may be an early sign of the disease and frequently develops as a reaction to increasing disability and social isolation. In elderly patients there is an increased risk of cognitive impairment and dementia. Parkinson's disease is more common in older people, with most cases occurring after the age of 60; when it is seen under the age of 45 years, it is called young onset PD.
The main motor symptoms are collectively called parkinsonism, or a "parkinsonian syndrome". Probably fewer than 5% of cases have a clear genetic origin, while secondary parkinsonism is due to known causes like drugs toxins and cerebrovascular disease. Many risks and protective factors have been investigated but the strongest evidence is for a reduced risk in tobacco smokers (although this may be due to individuals predisposed to PD being less prone to smoking addiction). The pathology of the disease is believed to be due to the accumulation of abnormal proteins in nerve cellss leading to eventual cell death. The aggregated protein forms microscopic inclusions known as Lewy bodies that can be found in the substantia nigra. Bradykinesia and rigidity result from loss of dopamine containing cells in the substantia nigra. The diagnosis of Parkinson’s disease is based on accurate history taking and a neurological examination. Neuroimaging including CT and MR imaging is useful to exclude other disorders masquerading as Parkinsons disease and dopamine transporter imaging (SPECT scans) can be helpful when the clinical diagnosis is uncertain.
L-dopa, the natural precursor of dopamine is the most effective treatment but other drugs including dopamine agonists, and selective monoamine oxidase inhibitiors are useful adjuvant treatments. As the disease progresses and dopaminergic neurons and a variety of other types of neurons continue to be lost, dopaminergic drugs remain effective for most motor symptoms related to dopamine deficiency but the response can vary from hour to hour (motor fluctuations) and most patients also experience the complication of involuntary writhing movements (dyskinesias). Surgery such as deep brain stimulation, and continuous infusions of dopaminergic drugs such as apomorphine and enteral levodopa infusion gels have been used to reduce motor symptoms in cases where oral medications have been ineffective in providing smooth control of dopamine-responsive motor symptoms or where severe tremor or dyskinesias cause disability. Medications to treat non-movement-related symptoms of PD, such as sleep disturbances, bowel and bladder symptoms, drooling of saliva and emotional problems are available. Diet and some forms of rehabilitation have also shown some effectiveness at improving symptoms. Research directions include investigations into new animal models of the disease and of the potential usefulness of gene therapy, stem cell implants and neuroprotective agents.
The disease is named after the English apothecary surgeon James Parkinson, who published the first detailed description in An Essay on the Shaking Palsy in 1817.[1][2] Several major organizations promote research and improvement of quality of life of those with the disease and their families. Public awareness campaigns include Parkinson's disease day (on the birthday of James Parkinson, 11 April) and the use of a red tulip as the symbol of the disease. People with parkinsonism who have increased the public's awareness of the condition include actor Michael J. Fox, Olympic cyclist Davis Phinney, England football player Ray Kennedy and professional boxer Muhammad Ali.
Classification
[edit]Parkinsonism is defined as the presence of bradykinesia (slowness of initiation of movement and motor decrement on repetitive movement) in combination with one of three other physical signs namely muscular ( lead pipe or cogwheel) rigidity, a rest tremor and postural instability.
Parkinson's disease is the most common form of parkinsonism and is usually defined as "primary" parkinsonism, meaning parkinsonism with no external identifiable cause.[3][4] These identifiable causes may include side of effects of drugs, toxins, infections, metabolic derangement and strategic brain lesions such as strokes. Several neurodegenerative disorders may also present with Parkinsonism and are sometimes referred to as atypical Parkinsonism or Parkinson’s plus syndromes. They include multiple system atrophy, progressive supranuclear palsy, corticobasal degeneration and dementia with Lewy bodies. There are also cases of Parkinsonism where the cause has been identified, including gene mutations.
Scientists sometimes refer to Parkinson’s disease as a synucleinopathy due to an abnormal accumulation of alpha-synuclein protein in the brain to distinguish it from other neurodegenarations such as Alzheimer's disease where the brain accumulates tau protein in the form of neurofibrillary tangles, and beta amyloid in the form of plaques.[5] Considerable clinical and pathological overlap exists between tauopathies and synucleinopathies. The most typical symptom of Alzheimer's disease is dementia.[5]
Dementia with Lewy bodies (DLB) is another synucleinopathy that has close pathological similarities with PD, and especially with the subset of PD cases with dementia. However, the relationship between PD and DLB is complex and still has to be clarified.[6] They may represent parts of a continuum with variable distinguishing clinical and pathological features or they may prove to be separate diseases.[6]
Signs and symptoms
[edit]
The most recognizable symptoms in Parkinson's disease affect the initiation and fluency of movement, giving rise to motor symptoms.[9] Non-motor symptoms, which include autonomic dysfunction, neuropsychiatric problems (mood, cognition, behavior or thought alterations), and sensory (especially altered sense of smell) and sleep difficulties, are also common. Some of these non-motor symptoms are often present at the time of diagnosis.[9]
Motor
[edit]Tremor is the most apparent and well-known symptom.[9] It is the most common; though around 30% of individuals with PD do not have tremor at disease onset, most develop it as the disease progresses.[9] It is usually a rest tremor: maximal when the limb is at rest and disappearing with voluntary movement and sleep.[9] It affects to a greater extent the most distal part of the limb and at onset typically appears in only a single arm or leg, becoming bilateral later.[9] Frequency of PD tremor is between 4 and 6 hertz (cycles per second). A feature of tremor is pill-rolling, the tendency of the index finger of the hand to get into contact with the thumb and perform together a circular movement.[9][10] The term derives from the similarity between the movement in people with PD and the early pharmaceutical technique of manually making pills.[10]
Bradykinesia (slowness of movement) is found in every case of PD, and is due to disturbances in motor planning of movement initiation, and associated with difficulties along the whole course of the movement process, from planning to initiation and execution of a movement.[9] Performance of sequential and simultaneous movement is impaired.[9] Bradykinesia is the most handicapping symptom of Parkinson’s disease leading to difficulties with everyday tasks such as dressing, feeding and bathing. It leads to particular difficulty in carrying out two independent motor activities at the same time and can be made worse by emotional stress or intercurrent illnesses. Paradoxically patients with Parkinsons disease can often ride a bicycle or climb stairs more easily than walk on a level. While most physicians may readily notice bradykinesia, formal assessment requires a patient to do repetitive movements with their fingers and feet.
Rigidity is stiffness and resistance to limb movement caused by increased muscle tone, an excessive and continuous contraction of muscles.[9] In parkinsonism the rigidity can be uniform (lead-pipe rigidity) or ratchety (cogwheel rigidity).[9][3][11][12] The combination of tremor and increased tone is considered to be at the origin of cogwheel rigidity.[13] Rigidity may be associated with joint pain; such pain being a frequent initial manifestation of the disease.[9] In early stages of PD, rigidity is often asymmetrical and it tends to affect the neck and shoulder muscles prior to the muscles of the face and extremities.[14] With the progression of the disease, rigidity typically affects the whole body and reduces the ability to move.
Postural instability is typical in later stages of the disease, leading to impaired balance and frequent falls,[15] and secondarily to bone fractures,[9] loss of confidence and reduced mobility. Instability is often absent in the initial stages, especially in younger people, especially prior to the development of bilateral symptoms.[3] Up to 40% may experience falls and around 10% may have falls weekly, with number of falls being related to the severity of PD.[9]
Other recognized motor signs and symptoms include gait and posture disturbances such as festination (rapid shuffling steps and a forward-flexed posture when walking with absent flexed arm swing. Freezing of gait (brief arrests when the feet seem to get stuck to the floor, especially on turning or changing direction)),[9] a slurred monotonous quiet voice,[16] mask-like face expression and handwriting that gets smaller and smaller are other common signs.[9]
Neuropsychiatric
[edit]Parkinson's disease can cause neuropsychiatric disturbances which can range from mild to severe. This includes disorders of cognition, mood, behaviour, and thought.[9]
Cognitive disturbances can occur in the initial stages of the disease and sometimes prior to diagnosis, and increase in prevalence with duration of the disease.[9][17]. The most common cognitive deficit in affected individuals is executive dysfunction, which can include problems with planning, cognitive flexibility, abstract thinking, rule acquisition, initiating appropriate actions and inhibiting inappropriate actions, and selecting relevant sensory information. Fluctuations in attention and slowed cognitive speed are among other cognitive difficulties. Memory is affected, specifically in recalling learned information. Nevertheless, improvement appears when recall is aided by cues. Visuospatial difficulties are also part of the disease, seen for example when the individual is asked to perform tests of facial recognition and perception of the orientation of drawn lines.[17]
A person with PD has two to six times the risk of dementia compared to the general population.[9][17] The prevalence of dementia increases with age and to a lesser degree the duration of the disease.[17] Dementia is associated with a reduced quality of life in people with PD and their caregivers, increased mortality, and a higher probability of needing nursing home care.[17]
Behavior and mood alterations are more common in PD without cognitive impairment than in the general population, and are usually present in PD with dementia. The most frequent mood difficulties are depression, apathy, anhedonia and anxiety.[9] Establishing the diagnosis of depression is complicated by the fact that the body language of depression may masquerade as PD including a sad expressionlees anxious face, a hang dog appearance, slow movement and monotonous speech.[18] Up to 30% of patients with PD may experience symptoms of anxiety, ranging from a generalized anxiety disorder to social fobia, panic disorders and obsessive compulsive disorders. They contribute to impaired quality of life and increased severity of motor symptoms such as on/off fluctuations or freezing episodes. Impulse control disorders including pathological gambling, compulsive sexual behaviour, binge eating, compulsive shopping and reckless generosity can be caused by medication, particularly orally active dopamine agonists. The dopamine dysregulation syndrome - with wanting of medication leading to overusage - is a rare complication of l-dopa use (Giovannoni, et al. 2000). Punding in which complicated repetitive aimless stereotyped behaviours occur for many hours is another iatrogenic disturbance.[9][19] Formed complex visual hallucinations or delusions occur in approximately 50% of people over a lifetime with PD and may be a harbinger for dementia. These range from "minor hallucinations" - sense of passage (something quickly passing beside the person) or presence (the perception of something/someone standing just to the side or behind the person) - to full blown vivid, formed visual hallucinations and paranoid ideation. Unlike schizophrenia, auditory hallucinations are uncommon, and are rarely described as voices. It is now believed that this disturbance is an integral part of the disease but anti-Parkinsonian drugs may be a risk factor. A psychosis with delusions and associated delirium is another recognized complication of drug treatment, but may also be caused by urinary tract infections as frequently occurs in the fragile elderly.[20][21] However, it is clear that this is not the only factor, and underlying brain pathology or changes in other neurotransmitters or their receptors (e.g., acetylcholine, serotonin) are also thought to play a role.
Other
[edit]In addition to cognitive and motor symptoms, PD can impair other body functions.
Sleep problems are a feature of the disease and can be worsened by medications.[9] Symptoms can manifest as daytime drowsiness (including sudden sleep attacks resembling narcolepsy), disturbances in REM sleep, or insomnia.[9] REM behavior disorder (RBD), in which patients act out dreams, sometimes injuring themselves or their bed partner, may begin many years before the development of motor or cognitive features of PD or DLB.
Alterations in the autonomic nervous system can lead to orthostatic hypotension (low blood pressure upon standing), oily skin and excessive sweating, urinary incontinence and altered sexual function.[9] Constipation and gastric dysmotility can be severe enough to cause discomfort and even endanger health.[22] Changes in perception may include an impaired sense of smell, disturbed vision, sensation of pain and paresthesia (skin tingling and numbness).[9] All of these symptoms can occur years before diagnosis of the disease.[9]
Causes
[edit]Parkinson's disease in most people is idiopathic (having no specific known cause). However, a small proportion of cases can be attributed to known genetic factors. Other factors have been associated with the risk of developing PD, but no causal relationships have been proven.
Environmental factors
[edit]Exposure to pesticides and a history of head injury have been linked with PD but the risk is modest. People who have never smoked cigarettes have an increased risk of developing PD while never drinking caffeinated beverages moderately increases risk. A high serum uric acid has also been found to reduce the risk of PD.
Genetics
[edit]
PD traditionally has been considered a non-genetic disorder; however, around 15% of individuals with PD have a first-degree relative who has the disease.[3] About 5% of people are now known to have forms of the disease that occur because of a mutation of one of several specific genes.[23]
Mutations in specific genes have been conclusively shown to cause PD. These genes code for alpha-synuclein (SNCA), parkin (PRKN), leucine-rich repeat kinase 2 (LRRK2 or dardarin), PTEN-induced putative kinase 1 (PINK1), DJ-1 and ATP13A2.[24][23] In most cases, people with these mutations will develop PD. With the exception of LRRK2, however, they account for only a small minority of cases of PD.[24] The most extensively studied PD-related genes are SNCA and LRRK2. Mutations in genes including SNCA, LRRK2 and glucocerebrosidase (GBA) have been found to be risk factors for sporadic PD. This means that harboring the mutation may not necessarily lead to the disease but puts the individual at an increased risk, often in combination with other risk factors. Mutations in GBA are known to cause Gaucher's disease.[23] Genome-wide association studies, which search for mutated alleles with low penetrance in sporadic cases, have now yielded many positive results.[25]
The role of the SNCA gene is important in PD because the alpha-synuclein protein is the main component of Lewy bodies.[23] Missense mutations of the gene (in which a single nucleotide is changed), and duplications and triplications of the locus containing it have been found in different groups with familial PD.[23] Missense mutations are rare.[23] On the other hand, multiplications of the SNCA locus account for around 2% of familial cases.[23] Multiplications have been found in asymptomatic carriers, which indicate that penetrance is incomplete or age-dependent.[23]
The LRRK2 gene (PARK8) encodes a protein called dardarin. The name dardarin was taken from a Basque word for tremor, because this gene was first identified in families from England and the north of Spain.[24] Mutations in LRRK2 are the most common known cause of familial and sporadic PD, accounting for approximately 5% of individuals with a family history of the disease and 3% of sporadic cases.[24][23] There are many mutations described in LRRK2, however unequivocal proof of causation only exists for a few.[23] LRRK2 mutation, especially the commonest (G2019S), may have penetrance as low as 26% in some populations, for example Ashkenazi Jews.
Several Parkinson-related genes are involved in the function of lysosomes, organelles that digest cellular waste products. It has been suggested that some forms of Parkinson may be caused by lysosome dysfunctions that reduce the ability of cells to break down alpha-synuclein.[26]
Pathology
[edit]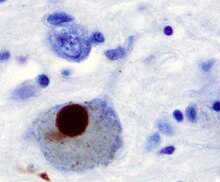
Anatomical
[edit]The basal ganglia, a group of brain structures innervated by the dopaminergic system, are the most seriously affected brain areas in PD.[27] The most important pathological lesion is cell death in the substantia nigra and, more specifically, the ventral (front) part of the pars compacta, affecting up to 70% of the cells by the time death occurs.[24]
Macroscopic alterations can be noticed on cut surfaces of the brainstem, where neuronal loss can be inferred from a reduction of neuromelanin pigmentation in the substantia nigra and locus coeruleus.[28] Several other brain regions show neuronal loss and Lewy bodies in many of the remaining nerve cells. Neuronal loss is accompanied by death of astrocytes (star-shaped glial cells) and activation of the microglia (another type of glial cell).[28]
Pathophysiology
[edit]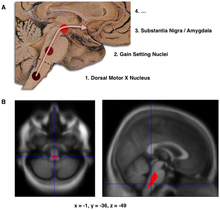
B. Localization of the area of significant brain volume reduction in initial PD compared with a group of participants without the disease in a neuroimaging study, which concluded that brain stem damage may be the first identifiable stage of PD neuropathology[29]
The primary symptoms of PD result from greatly reduced activity of dopamine-secreting cells caused by cell death in the pars compacta region of the substantia nigra.[27]
There are five major pathways in the brain connecting other brain areas with the basal ganglia. These are known as the motor, oculo-motor, associative, limbic and orbitofrontal circuits, with names indicating the main projection area of each circuit.[27] All of them are affected in PD, and their disruption explains many of the symptoms of the disease since these circuits are involved in a wide variety of functions including movement, attention and learning.[27] Scientifically, the motor circuit has been examined the most intensively.[27]
A particular conceptual model of the motor circuit and its alteration with PD has been of great influence since 1980, although some limitations have been pointed out which have led to modifications.[27] In this model, the basal ganglia normally exert a constant inhibitory influence on a wide range of motor systems, preventing them from becoming active at inappropriate times. When a decision is made to perform a particular action, inhibition is reduced for the required motor system, thereby releasing it for activation. Dopamine acts to facilitate this release of inhibition, so high levels of dopamine function tend to promote motor activity, while low levels of dopamine function, such as occur in PD, demand greater exertions of effort for any given movement. Thus, the net effect of dopamine depletion is to produce hypokinesia, an overall reduction in motor output.[27] Drugs that are used to treat PD, conversely, may produce excessive dopamine activity, allowing motor systems to be activated at inappropriate times and thereby producing dyskinesias.[27]
Brain cell death
[edit]There is speculation of several mechanisms by which the brain cells could be lost.[30] One mechanism consists of an abnormal accumulation of the protein alpha-synuclein bound to ubiquitin in the damaged cells. This insoluble protein accumulates inside neurones forming inclusions called Lewy bodies.[24][31] According to the Braak staging, a classification of the disease based on pathological findings, Lewy bodies first appear in the olfactory bulb, medulla oblongata and pontine tegmentum; individuals at this stage may be asymptomatic or may have prodromal non-motor symptoms (such as loss of sense of smell, sleep and some automatic dysfunction). As the disease progresses, Lewy bodies later develop in the substantia nigra, areas of the midbrain and basal forebrain, and, finally, the neocortex.[24] These brain sites are the main places of neuronal degeneration in PD; however, Lewy bodies may not cause cell death and they may be protective (with the abnormal protein sequestered or walled off).[30][31] Other forms of alpha-synuclein (e.g., oligomers) that are not aggregated in Lewy bodies and Lewy neurites may actually be the toxic forms of the protein. In people with dementia, a generalized presence of Lewy bodies is common in cortical areas. Neurofibrillary tangles and senile plaques, characteristic of Alzheimer's disease, are not common unless the person is demented.[28]
Other cell-death mechanisms include proteosomal and lysosomal system dysfunction and reduced mitochondrial activity.[30] Iron accumulation in the substantia nigra is typically observed in conjunction with the protein inclusions. It may be related to oxidative stress, protein aggregation and neuronal death, but the mechanisms are not fully understood.[32]
Diagnosis
[edit]
A physician will diagnose PD from the medical history and a neurological examination.[9] There is no lab test that will clearly identify the disease, but MRI scans are sometimes used to rule out disorders that could give rise to similar symptoms. People may be given levodopa and resulting relief of motor impairment tends to confirm diagnosis but this is not a reliable test in early disease. The finding of Lewy bodies in the midbrain on autopsy is usually considered proof that the person had PD. The progress of the illness over time may reveal it is not PD, and some authorities recommend that the diagnosis be periodically reviewed.[9][33]
Other causes that can secondarily produce a parkinsonian syndrome are multiple cerebral infarction and drug-induced parkinsonism.[33] Parkinson plus syndromes such as progressive supranuclear palsy and multiple system atrophy must be ruled out.[9] Anti-Parkinson's medications are typically less effective at controlling symptoms in Parkinson plus syndromes.[9] Faster progression rates, early cognitive dysfunction or postural instability, minimal tremor or symmetry at onset may indicate a Parkinson plus disease rather than PD itself.[34] Genetic forms with an autosomal dominant or recessive pattern of inheritance are sometimes referred to as familial Parkinson's disease or familial parkinsonism.[3]
Medical organizations have created diagnostic criteria to ease and standardize the diagnostic process, especially in the early stages of the disease. The most widely known criteria come from the UK Queen Square Brain Bank for Neurological Disorders and the U.S. National Institute of Neurological Disorders and Stroke.[9] The Queen Square Brain Bank criteria require slowness of movement (bradykinesia) plus either rigidity, resting tremor, or postural instability. Other possible causes for these symptoms need to be ruled out. Finally, three or more of the following supportive features are required during onset or evolution: unilateral onset, tremor at rest, progression in time, asymmetry of motor symptoms, response to levodopa for at least five years, clinical course of at least ten years and appearance of dyskinesias induced by the intake of excessive levodopa.[9] Accuracy of diagnostic criteria evaluated at autopsy is 75–90%, with specialists such as neurologists having the highest rates.[9] Very recently, a task force of the International Parkinson and Movement Disorder Society (MDS) has proposed diagnostic criteria for Parkinson’s disease[35] as well as research criteria for the diagnosis of prodromal disease,[36] but these will require validation against the more established criteria.
Computed tomography (CT) and conventional magnetic resonance imaging (MRI) brain scans of people with PD usually appear normal.[37] These techniques are nevertheless useful to rule out other diseases that can be secondary causes of parkinsonism, such as basal ganglia tumors, vascular pathology and hydrocephalus.[37] A specific technique of MRI, diffusion MRI, has been reported to be useful at discriminating between typical and atypical parkinsonism, although its exact diagnostic value is still under investigation.[37] Dopaminergic function in the basal ganglia can be measured with different PET and SPECT radiotracers. Examples are ioflupane (123I) (trade name DaTSCAN) and iometopane (Dopascan) for SPECT or fluoro-L-dopa (18F)[37] and DTBZ[38] for PET. A pattern of reduced dopaminergic activity in the basal ganglia can aid in diagnosing[37]and distinguish it from drug-induced parkinsonism. Reduced dopaminergic activity is, however, also seen in the Parkinson-plus disorders so they are not reliable in distinguishing PD from other neurodegenerative causes of parkinsonism and therefore of limited use in this clinical situation.
Prevention
[edit]Exercise in middle age may slightly reduce the risk of PD later in life.[39] Caffeine also appears protective with a greater decrease in risk occurring with a larger intake of caffeinated beverages such as coffee.[40] Although tobacco smoke causes adverse health effects, decreases life expectancy and quality of life, it may reduce the risk of PD by a third when compared to non-smokers.[41] The basis for this effect is not known, but possibilities include an effect of nicotine as a dopamine stimulant.[41][42] Tobacco smoke contains compounds that act as MAO inhibitors that also might contribute to this effect.[43] However, recently it has been suggested that the negative association with smoking is not protective but is due to an inherent difference in the propensity of patients destined to develop PD to become addicted to nicotine.[44]
Antioxidants, such as vitamins C and E, have been proposed to protect against the disease but results of studies have been contradictory and no positive effect has been proven.[41] The results regarding fat and fatty acids have been contradictory, with various studies reporting protective effects, risk-increasing effects or no effects.[41] Also, there have been preliminary indications of a possible protective role of estrogens, anti-inflammatory drugs, certain calcium channel blocking drugs () and higher levels of uric acid ().[41]
Management
[edit]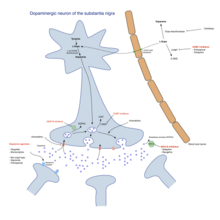
There is no cure for PD, but medications, surgery and physical treatment can provide relief, and are much more effective than those available for other neurological disorders like Alzheimer’s disease, motor neurone disease, the Parkinson plus syndromes and multiple sclerosis. The main families of drugs useful for treating motor symptoms are L-DOPA (now always combined with a dopa decarboxylase inhibitor which does not cross the blood–brain barrier), and sometimes combined with a COMT inhibitor, dopamine agonists and MAO-B inhibitors.[45] The stage of the disease determines which group is most useful. Three stages are usually distinguished: an initial stage in which the individual with PD has already developed some disability requiring pharmacological treatment, a second stage associated with the development of motor complications related to levodopa usage, and a third stage when non-dopaminergic symptoms (motor and non-motor) may predominate.[45] Treatment in the initial stage aims for an optimal tradeoff between good symptom control and side-effects resulting from improvement of dopaminergic function. The start of levodopa (or L-DOPA) treatment may be delayed by using other medications such as MAO-B inhibitors and dopamine agonists, in the hope of delaying the onset of dyskinesias.[45] However, levodopa remains the most effective treatment for PD and should not be delayed in patients whose quality of life is impaired. Indeed, dyskinesias correlate more strongly with duration and severity of the disease than duration of levodopa treatment, so delaying this therapy may not really provide much longer dyskinesia-free time than earlier use (). In the second stage the aim is to reduce symptoms while controlling fluctuations of the response to medication. Sudden withdrawals from medication or overuse have to be managed.[45] When medications are not enough to adequately control symptoms, surgery such as deep brain stimulation, and infusion therapies using pumps can be of use.[46] The third stage presents many challenging problems requiring a variety of treatments for psychiatric symptoms, orthostatic hypotension, bladder dysfunction, etc.. In the final stages of the disease, palliative care is provided to improve quality of life.[47]
Levodopa
[edit]Levodopa has been the most widely used treatment for over 30 years.[45] L-DOPA is converted into dopamine in the dopaminergic and possibly other (e.g., serotonergic) neurons by dopa decarboxylase.[45] Since motor symptoms are produced by a lack of dopamine in the substantia nigra, the administration of L-DOPA temporarily diminishes the motor symptoms.[45]
Only 5–10% of L-DOPA crosses the blood–brain barrier. The remainder is often metabolized to dopamine elsewhere, causing a variety of side effects including nausea, vomiting and orthostatic hypotension.[45] Carbidopa and benserazide are peripheral dopa decarboxylase inhibitors,[45] which help to prevent the metabolism of L-DOPA before it reaches the dopaminergic neurons, therefore reducing side effects and increasing bioavailability. They are generally given as combination preparations with levodopa.[45] Existing preparations are carbidopa/levodopa (co-careldopa) and benserazide/levodopa (co-beneldopa). There are controlled release versions of levodopa. Older controlled-release levodopa preparations have poor and unreliable absorption and bioavailability and have not shown an increased control of motor symptoms or motor complications when compared to immediate release preparations.[45][48] A newer extended-release levodopa preparation[49]does seem to be more effective in controlling motor fluctuations but in many patients problems persist. Intestinal infusions of levodopa (Duodopa) can result in striking improvements in motor fluctuations compared to oral levodopa due to insufficient uptake caused by gastroparesis.[50] Other oral, longer acting formulations are under study currently and other formulations of levodopa (inhaled, transdermal) are under development.
Tolcapone inhibits the COMT enzyme, which degrades dopamine, thereby prolonging the effects of levodopa.[45] It has been used to complement levodopa; however, its usefulness is limited by possible side effects such as liver damage.[45] Another COMT-inhibitor, entacapone, has not been shown to cause significant alterations of liver function but is less efficacious than tolcapone.[45] Licensed preparations of entacapone contain entacapone alone or in combination with carbidopa and levodopa.[45]
Levodopa preparations frequently lead in the long term to the development of motor complications characterized by involuntary movements called dyskinesias and fluctuations in the response to medication.[45] When this occurs a person with PD can change from phases with good response to medication and few symptoms ("on" state), to phases with no response to medication and significant motor symptoms ("off" state).[45] For this reason, levodopa doses are kept as low as possible while maintaining functionality.[45] A former strategy to reduce motor complications was to withdraw L-DOPA medication for some time. This is discouraged now, since it can bring dangerous side effects such as neuroleptic malignant syndrome.[45] Most people with PD will eventually need levodopa and later develop motor side effects.[45]
Dopamine agonists
[edit]Several dopamine agonists that bind to dopaminergic post-synaptic receptors in the brain have similar effects to levodopa.[45] These were initially used for individuals experiencing on-off fluctuations and dyskinesias as a complementary therapy to levodopa; they are now mainly used on their own as an initial therapy for motor symptoms with the aim of delaying motor complications.[45][51]. Like levodopa, these can improve all of the dopaminergic motor symptoms but they are generally less effective than levodopa.When used in late PD they are useful at reducing the off periods.[45] Dopamine agonists include bromocriptine, pergolide, pramipexole, ropinirole, piribedil, cabergoline, apomorphine, lisuride and rotigotine. The most commonly used oral dopamine agonists are pramipexole and ropinerole. Rotigotine is used as a transdermal patch.
Dopamine agonists can produce significant side effects including orthostatic hypotension, drowsiness, hallucinations, insomnia, nausea and constipation.[45] Sometimes side effects appear even at a minimal clinically effective dose, leading the physician to search for a different drug.[45] Compared with levodopa, dopamine agonists may delay motor complications of medication use but are less effective at controlling symptoms.[45] They tend to be more expensive than levodopa.[3] Dyskinesias due to dopamine agonists are rare in younger people who have PD, but along with other side effects, become more common with age at onset.[3] Thus dopamine agonists may be preferred as initial treatment in younger-onset patients, as opposed to levodopa for later onset.[3] However, recent studies have emphasized little advantage to so-called "levodopa sparing" approaches (e.g., dopamine agonists, MAO-B inhibitors) in early disease.[52] Agonists have been related to impulse control disorders, particularly pathological gambling[19] and discontinuing dopamine agonists may be associated with "dopamine agonist withdrawal syndrome",[53] with symptoms similar to withdrawal from narcotics, and apathy.[54]
MAO-B inhibitors
[edit]MAO-B inhibitors (selegiline and rasagiline) increase the level of dopamine in the basal ganglia by blocking its metabolism. These agents inhibit the enzyme, monoamine oxidase B (MAO-B) which breaks down dopamine secreted by the dopaminergic neurons. The reduction in MAO-B activity results in increased L-DOPA in the striatum.[45] Like dopamine agonists, MAO-B inhibitors used as monotherapy improve motor symptoms and delay the need for levodopa in early disease, but are clearly less effective than levodopa. There are few studies of drug effectiveness in the advanced stage, although results suggest that they are useful to reduce fluctuations between on and off periods.[45] There remains a question whether these MAO-B inhibitors may slow the progression of the disease. This effect has not been proven and is likely very modest is it exists at all.
Other drugs
[edit]Other drugs such as amantadine and anticholinergics may be useful as treatment of motor symptoms. However, because they are old drugs the evidence from modern clinical trials supporting them lacks quality, so they are rarely first choice treatments.[45] Amatadine is now most often used to control dyskinesias in many patients. In addition to motor symptoms, PD is accompanied by a diverse range of symptoms. A number of drugs have been used to treat some of these problems.[55] Examples are the use of clozapine and quetiapine for psychosis, cholinesterase inhibitors for dementia, and modafinil for daytime sleepiness.[55][56]
Surgery
[edit]
Treating motor symptoms with surgery was once a common practice, but since the introduction of L-DOPA in 1969, the number of operations declined.[57] Studies in the past few decades have led to great improvements in surgical techniques, so that surgery is again being used in people with advanced PD for whom drug therapy no longer sufficiently controls symptoms due to motor fluctuations, and increasingly in patients with less advanced disease.[57] Surgery for PD can be divided in two main groups: lesional and deep brain stimulation (DBS). Target areas for DBS or lesions include the thalamus, the globus pallidus or the subthalamic nucleus and depend on the main clinical symptoms involved.[57] Deep brain stimulation (DBS) of the subthalamic nucleus is the most commonly used surgical treatment, developed in the 1980s by Alim-Louis Benabid and others. It involves the implantation of a medical device called a neurostimulator which sends electrical impulses to specific parts of the brain. DBS is recommended for people who have PD with motor fluctuations and tremor inadequately controlled by medication, or to those who are intolerant to medication, as long as they do not have severe neuropsychiatric problems.[46] Other, less common, surgical therapies involve intentional formation of lesions to suppress overactivity of specific subcortical areas. For example, pallidotomy involves surgical destruction (a lesion) of the globus pallidus to control dyskinesia,[57] and thalamotomy lesions a region in the thalamus to control tremor. A new technique of lesioning brain areas such as the thalamus without opening the skull uses the technique of focused ultrasound and another uses gamma irradiation (gamma-knife). A major advantage of DBS procedures over these and older lesioning techniques is that they can be applied much more safely to both sides of the brain (bilateral procedures) and is more reversible by switching the device on and off. New developments in DBS include manufacturing closed loop systems in which deep brain electrodes simultaneously pick up local field potentials to respond with the appropriate electrical signal.
Rehabilitation
[edit]Exercise programs are recommended in people with Parkinson's disease.[39] There is some evidence that speech or mobility problems can improve with rehabilitation, although studies are scarce and of low quality.[58][59] Regular physical exercise with or without physiotherapy can be beneficial to maintain and improve mobility, flexibility, strength, gait speed, and quality of life.[59] When an exercise program is performed under the supervision of a physiotherapist, there are more improvements in motor symptoms, mental and emotional functions, daily living activities, and quality of life compared to a self-supervised exercise program at home.[60] In terms of improving flexibility and range of motion for people experiencing rigidity, generalized relaxation techniques such as gentle rocking have been found to decrease excessive muscle tension. Other effective techniques to promote relaxation include slow rotational movements of the extremities and trunk, rhythmic initiation, diaphragmatic breathing, and meditation techniques.[61] As for gait and addressing the challenges associated with the disease such as hypokinesia (slowness of movement), shuffling and decreased arm swing; physiotherapists have a variety of strategies to improve functional mobility and safety. Areas of interest with respect to gait during rehabilitation programs focus on but are not limited to improving gait speed, base of support, stride length, trunk and arm swing movement. Strategies include utilizing assistive equipment (pole walking and treadmill walking), verbal cueing (manual, visual and auditory), exercises (marching and PNF patterns) and altering environments (surfaces, inputs, open vs. closed).[62] Strengthening exercises have shown improvements in strength and motor function for people with primary muscular weakness and weakness related to inactivity with mild to moderate PD. However, reports show a significant interaction between strength and the time the medications was taken. Therefore, it is recommended that people with PD should perform exercises 45 minutes to one hour after medications, when they are at their best.[63] Also, due to the forward flexed posture, and respiratory dysfunctions in advanced PD, deep diaphragmatic breathing exercises are beneficial in improving chest wall mobility and vital capacity.[64] Exercise may improve constipation.[22]
One of the most widely practiced treatments for speech disorders associated with Parkinson's disease is the Lee Silverman voice treatment (LSVT).[58][65] Speech therapy and specifically LSVT may improve speech.[58] Occupational therapy (OT) aims to promote health and quality of life by helping people with the disease to participate in as many of their daily living activities as possible.[58] There have been few studies on the effectiveness of OT and their quality is poor, although there is some indication that it may improve motor skills and quality of life for the duration of the therapy.[58][66]
Palliative care
[edit]Palliative care is specialized medical care for people with serious illnesses, including parkinsonism. The goal of this speciality is to improve quality of life for both the person suffering from PD and the family by providing relief from the symptoms, pain, and stress of illnesses.[67] As PD is not a curable disease, all treatments are focused on slowing decline and improving quality of life, and are therefore palliative in nature.[68]
Palliative care should be involved earlier, rather than later in the disease course.[69][70] Palliative care specialists can help with physical symptoms, emotional factors such as loss of function and jobs, depression, fear, and existential concerns.[69][70][71]
Along with offering emotional support to both the patient and family, palliative care serves an important role in addressing goals of care. People with parkinsonism may have many difficult decisions to make as the disease progresses such as wishes for feeding tube, non-invasive ventilator, and tracheostomy; wishes for or against cardiopulmonary resuscitation; and when to use hospice care.[68] Palliative care team members can help answer questions and provide guidance around these complex and emotional topics.[70][72]
Other treatments
[edit]Muscles and nerves that control the digestive process may be affected by PD, resulting in constipation and gastroparesis (food remaining in the stomach for a longer period than normal).[22] A balanced diet, based on periodical nutritional assessments, is recommended and should be designed to avoid weight loss or gain and minimize consequences of gastrointestinal dysfunction.[22] As the disease advances, swallowing difficulties (dysphagia) may appear. In such cases it may be helpful to use thickening agents for liquid intake and an upright posture when eating, both measures reducing the risk of choking. Gastrostomy to deliver food directly into the stomach is possible in severe cases.[22]
L-DOPA and proteins use the same transportation system in the intestine and the blood–brain barrier, thereby competing for access.[22] When they are taken together, this results in a reduced effectiveness of the drug.[22] Therefore, when L-DOPA is used, excessive protein consumption may reduce medication efficacy in some patients. In advanced stages, additional intake of low-protein products such as bread or pasta is recommended for similar reasons.[22] If dietary protein is determined to reduce levodopa efficacy, strategies to minimize interaction with protein include ingestion of levodopa 60 minutes before meals or 2 hours after meals.[22] At the same time, regimens for PD restrict proteins during breakfast and lunch, allowing protein intake in the evening.[22] Food in the stomach and constipation both slow stomach emptying which delays or reduces L-DOPA access to the upper small intestine where it is absorbed, resulting in poorer responses to individual doses.
Repetitive transcranial magnetic stimulation temporarily improves levodopa-induced dyskinesias.[73] Its usefulness in PD is an open research topic,[74] although recent studies have shown no effect by rTMS.[75] Several nutrients have been proposed as possible treatments; however there is no evidence that vitamins, food additives or orally ingested and intravenous glutathione improve symptoms.[76] There is no evidence to substantiate that acupuncture and practice of Qigong, or T'ai chi, have any effect on the course of the disease or symptoms. Further research on the viability of Tai chi for balance or motor skills are necessary.[77][78][79] Fava beans and the cowhage (Mucuna pruriens) are natural sources of levodopa and are used by people with PD. While they have shown some effectiveness in clinical trials,[80] their intake is not free of risks, particularly since the quantities of levodopa received in these formulations are quite variable.[81][82]
Prognosis
[edit]
| no data < 5 5–12.5 12.5–20 20–27.5 27.5–35 35–42.5 | 42.5–50 50–57.5 57.5–65 65–72.5 72.5–80 > 80 |
PD invariably progresses with time. A severity rating method known as the Unified Parkinson's Disease Rating Scale (UPDRS) is the most commonly used metric for clinical study. A modified version known as the MDS-UPDRS is incresaingly being used. An older scaling method known as the Hoehn and Yahr scale (originally published in 1967), and a similar scale known as the Modified Hoehn and Yahr scale, have also been commonly used. The Hoehn and Yahr scale defines five basic stages of progression; it emphasizes gait and posture instability levodopa response, although ratings for the status of on and off medication can be applied.
Motor symptoms, if not treated, advance in linear fashion. Untreated individuals lose independent ambulation after an average of eight years and are bedbound and dependent on others for everyday activities like dressing feeding and bathing after ten years.[83] However, it is uncommon to find untreated people nowadays. Medication has improved quality of life considerably and probably life expectancy by reducing early deaths.[83] In people taking levodopa, the progression time of symptoms to a stage of high dependency from caregivers may be over 15 years.[83] However, it is hard to predict what course the disease will take for a given individual.[83] Age is the best predictor of disease progression.[30] The rate of motor decline is greater in those with less impairment at the time of diagnosis, while cognitive impairment is more frequent in those who are over 70 years of age at symptom onset.[30]
Since current therapies improve many of the motor symptoms, disability at present is mainly related to non-motor features of the disease as well as the non-dopaminergic motor features.[30] Nevertheless, the relationship between disease progression and disability is not linear. Disability is initially related to motor symptoms.[83] As the disease advances, disability is more related to motor symptoms that do not respond adequately to medication, such as swallowing/speech difficulties, and gait/balance problems; and also to motor complications, which appear in up to 50% of individuals after 5 years of levodopa usage.[83] Finally, after ten years most people with the disease have autonomic disturbances, sleep problems, mood alterations and cognitive decline.[83] All of these symptoms, especially cognitive decline, greatly increase disability.[30][83]
The life expectancy of people with PD is reduced.[83] Mortality ratios are around twice those of unaffected people.[83] Cognitive decline and dementia, old age at onset, a more advanced disease state and presence of swallowing problems are all mortality risk factors. On the other hand, a disease pattern mainly characterized by tremor as opposed to rigidity predicts an improved survival.[83] Death from aspiration pneumonia is twice as common in individuals with PD as in the healthy population.[83]
In 2013 PD resulted in about 103,000 deaths globally, up from 44,000 deaths in 1990.[84] The death rate increased from an average of 1.5 to 1.8 per 100,000 during that time.[84]
Epidemiology
[edit]PD is the second most common neurodegenerative disorder after Alzheimer's disease and affects approximately seven million people globally and one million people in the United States.[15][41] The proportion in a population at a given time is about 0.3% in industrialized countries. PD is more common in the elderly and rates rises from 1% in those over 60 years of age to 4% of the population over 80.[41] The mean age of onset is around 60 years, although 5–10% of cases, classified as young onset PD, begin between the ages of 20 and 50.[3] There is also some evidence that the incidence of the disease reduces in the ninth decade of life. PD may be less prevalent in those of African and Asian ancestry, although this finding is disputed.[41] Most, but not all, studies show that it is more common in men than women, but others failed to detect any differences between the two sexes.[41] The number of new cases per year of PD is between 8 and 18 per 100,000 person–years.[41]
History
[edit]
Several early sources, including an Egyptian papyrus, an Ayurvedic medical treatise, the Bible, and Galen's Leonardo da Vinci's writings, describe symptoms suggestive of PD.[85] After Galen there are no references unambiguously related to PD until the 17th century.[85] In the 17th and 18th centuries, several authors wrote about elements of the disease, including Sylvius, Gaubius, Hunter and Chomel.[85][86][87]
In 1817 an English doctor, James Parkinson, published his essay reporting six cases of paralysis agitans.[88] An Essay on the Shaking Palsy described the characteristic resting tremor, abnormal posture and gait, paralysis and diminished muscle strength, and the way that the disease progresses over time.[1][89] Early neurologists who made further additions to the knowledge of the disease include Trousseau, Gowers, Kinnier Wilson and Erb, and Jean-Martin Charcot whose studies between 1868 and 1881 were a landmark in the understanding of the disease.[88] Among other advances, he made the distinction between rigidity, weakness and bradykinesia.[88] He also championed the renaming of the disease in honor of James Parkinson.[88]
In 1912 Frederic Lewy described microscopic particles in affected brains, later named "Lewy bodies".[88] In 1919 Konstantin Tretiakoff reported that the substantia nigra was the main cerebral structure affected, but this finding was not widely accepted until it was confirmed by further studies published by Rolf Hassler in 1938.[88] The underlying biochemical changes in the brain were identified in the 1950s, due largely to the work of Arvid Carlsson on the neurotransmitter dopamine and Oleh Hornykiewicz on its role on PD.[90] In 1997, alpha-synuclein was found to be the main component of Lewy bodies by Spillantini, Trojanowski, Goedert and others.[31]
Anticholinergics and surgery (lesioning of the corticospinal pathway or some of the basal ganglia structures) were the only treatments until the arrival of levodopa, which reduced their use dramatically.[86][91] Levodopa was first synthesized in 1911 by Casimir Funk, but it received little attention until the mid 20th century.[90] It entered clinical practice in 1967 and brought about a revolution in the management of PD.[90][92]
Society and culture
[edit]Cost
[edit]
The costs of PD to society are high, but precise calculations are difficult due to methodological issues in research and differences between countries.[93] The annual cost in the UK is estimated to be between 449 million and 3.3 billion pounds, while the cost per patient per year in the U.S. is probably around $10,000 and the total burden around 23 billion dollars.[93] The largest share of direct cost comes from inpatient care and nursing homes, while the share coming from medication is substantially lower.[93] Indirect costs are high, due to reduced productivity and the burden on caregivers.[93] In addition to economic costs, PD reduces quality of life of those with the disease and their caregivers.[93]
Advocacy
[edit]11 April, the birthday of James Parkinson, has been designated as Parkinson's disease day.[88][94] A red tulip was chosen by international organizations as the symbol of the disease in 2005: it represents the James Parkinson Tulip cultivar, registered in 1981 by a Dutch horticulturalist.[94] Advocacy organizations include the National Parkinson Foundation, which has provided more than $180 million in care, research and support services since 1982,[95] Parkinson's Disease Foundation, which has distributed nearly $110 million for research and nearly $47 million for education and advocacy programs since its founding in 1957 by William Black;[96][97] the American Parkinson Disease Association, founded in 1961;[98] Parkinson’s UK, which has been helping people with Parkinson’s in the UK for over 40 years, and the European Parkinson's Disease Association, founded in 1992.[99]
Notable cases
[edit]
Actor Michael J. Fox has PD and has greatly increased the public awareness of the disease.[100] After diagnosis, Fox embraced his Parkinson's in television roles, sometimes acting without medication, in order to further illustrate the effects of the condition. He has written two autobiographies in which his fight against the disease plays a major role,[101] and appeared before the United States Congress without medication to illustrate the effects of the disease.[101] The Michael J. Fox Foundation aims to develop a cure for Parkinson's disease.[101] Fox received an honorary doctorate in medicine from Karolinska Institutet for his contributions to research in Parkinson's disease.[102]
Professional cyclist and Olympic medalist Davis Phinney, who was diagnosed with young onset Parkinson's at age 40, started the Davis Phinney Foundation in 2004 to support Parkinson's research, focusing on quality of life for people with the disease.[103][104][105]
Muhammad Ali showed signs of Parkinson's when he was 38, but was not diagnosed until he was 42, and has been called the "world's most famous Parkinson's patient".[106] Whether he has PD or a parkinsonism related to boxing is unresolved.[107][108] Ray Kennedy the Arsenal and Liverpool football player developed PD towards the end of his professional football career at the age of 35. Retrospective examination of television footage confirmed that he had physical signs in one arm while he was still playing football at a high level(Lees 1992).
Research
[edit]There is little prospect of dramatic new PD treatments expected in the next 5 years.[109] Currently active research directions include the search for new animal models of the disease and studies of the potential usefulness of gene therapy, stem cell transplants and neuroprotective agents.[30]
Animal models
[edit]PD is not known to occur naturally in any species other than humans, although animal models which show some features of the disease are used in research. The neurotoxin 6-hydroxydopamine, also known as 6-OHDA, creates a model of PD in rats by targeting and destroying dopaminergic neurons in the nigrostriatal pathway when injected into the substantia nigra or the striatum.[110] The appearance of parkinsonian symptoms in a group of drug addicts in the early 1980s who consumed a contaminated batch of the synthetic opiate MPPP led to the discovery of the chemical MPTP as an agent that causes a parkinsonian syndrome in non-human primates as well as in humans.[111] Other predominant toxin-based models employ the insecticide rotenone, the herbicide paraquat and the fungicide maneb.[112] Models based on toxins are most commonly used in primates. Transgenic rodent models that replicate various aspects of PD have been developed.[113]
Gene therapy
[edit]Gene therapy typically involves the use of a non-infectious virus (i.e., a viral vector such as the adeno-associated virus) to shuttle genetic material into a part of the brain. The gene used leads to the production of proteins that could affect symptoms of PD in a variety of ways including increasing dopamine production, stimulating the sprouting or regeneration of remaining dopamine cells by trophic factors, or causing neurons to change their transmitter function in hopes of normalizing basal ganglia network activity.[30][114] In 2010 there were four clinical trials using gene therapy in PD.[30] There have not been important adverse effects in these trials although the clinical usefulness of gene therapy is still unknown.[30] One of these reported positive results in 2011,[115] but the company filed for bankruptcy in March 2012.[116] More recently, gene therapy for the trophic factor neurturin, injected into both the striatum and substantia nigra, failed to show benefit in a well-designed randomized controlled clinical trial.[117]
Neuroprotective treatments
[edit]
Investigations on neuroprotection are at the forefront of PD research. Several molecules have been proposed as potential treatments.[30] However, none of them have been conclusively demonstrated to reduce degeneration.[30] Agents currently under investigation include, calcium channel blockers (isradipine) and growth factors (GDNF).[30] Preclinical research also targets alpha-synuclein.[109] Both active and passive methods of immunizing against alpha-synuclein are being actively pursued.[118]
Neural transplantation
[edit]Since early in the 1980s, fetal, porcine, carotid or retinal tissues have been used in cell transplants, in which cells are injected into the striatum in the hope that they will incorporate themselves into the brain in a way that replaces the dopamine-producing cells that have been lost.[30] Although there was initial evidence of mesencephalic dopamine-producing cell transplants being beneficial, double-blind trials to date indicate that cell transplants fail to provide adequate benefit in the majority of patients, particularly in these trials, although individual patients (often younger and with milder disease) have shown marked prolonged improvement.[30] An additional significant problem was the development of graft-induced dyskinesias that did not require levodopa or other dopaminergic medications to be maintained.[119] Stem cell transplants are a recent research target, because stem cells are easy to manipulate and stem cells transplanted into the brains of rodents and monkeys have been found to survive and reduce behavioral abnormalities.[30][120] Nevertheless, use of fetal stem cells is controversial.[30] It has been proposed that effective treatments may be developed in a less controversial way by use of induced pluripotent stem cells taken from adults.[30] It remains uncertain whether early ‘physiological’ replacement of the damaged nigrostriatal dopamine pathway will correct all the symptoms and signs of Parkinson’s disease.[121]
References
[edit]- ^ a b "An Essay on the Shaking Palsy".
- ^ Shulman JM, De Jager PL, Feany MB (February 2011) [25 October 2010]. "Parkinson's disease: genetics and pathogenesis". Annual Review of Pathology. 6: 193–222. doi:10.1146/annurev-pathol-011110-130242. PMID 21034221.
- ^ a b c d e f g h i Cite error: The named reference
pmid15172778was invoked but never defined (see the help page). - ^ Schrag A (2007). "Epidemiology of movement disorders". In Tolosa E, Jankovic JJ (eds.). Parkinson's disease and movement disorders. Hagerstown, Maryland: Lippincott Williams & Wilkins. pp. 50–66. ISBN 978-0-7817-7881-7.
- ^ a b Galpern WR, Lang AE (March 2006) [17 February 2006]. "Interface between tauopathies and synucleinopathies: a tale of two proteins". Annals of Neurology. 59 (3): 449–458. doi:10.1002/ana.20819. PMID 16489609. S2CID 19395939.
- ^ a b Aarsland D, Londos E, Ballard C (April 2009) [28 January 2009]. "Parkinson's disease dementia and dementia with Lewy bodies: different aspects of one entity". Int. Psychogeriatr. 21 (2): 216–219. doi:10.1017/S1041610208008612. PMID 19173762. S2CID 5433020.
- ^ Photo by Arthur Londe from Nouvelle Iconographie de la Salpètrière, vol. 5., p.226
- ^ Charcot, Jean-Martin; Sigerson, George (1879). Lectures on the diseases of the nervous system (Second ed.). Philadelphia: Henry C. Lea. p. 113.
The strokes forming the letters are very irregular and sinuous, whilst the irregularities and sinuosities are of a very limited width. (...) the down-strokes are all, with the exception of the first letter, made with comparative firmness and are, in fact, nearly normal — the finer up-strokes, on the contrary, are all tremulous in appearance (...).
- ^ a b c d e f g h i j k l m n o p q r s t u v w x y z aa ab ac ad ae af ag Cite error: The named reference
Jankovic2008was invoked but never defined (see the help page). - ^ a b Cooper G, Eichhorn G, Rodnitzky RL (2008). "Parkinson's disease". In Conn PM (ed.). Neuroscience in medicine. Totowa, NJ: Humana Press. pp. 508–512. ISBN 978-1-60327-454-8.
- ^ Banich MT, Compton RJ (2011). "Motor control". Cognitive neuroscience. Belmont, CA: Wadsworth, Cengage learning. pp. 108–144. ISBN 978-0-8400-3298-0.
- ^ Longmore M, Wilkinson IB, Turmezei T, Cheung CK (4 January 2007). Oxford Handbook of Clinical Medicine. Oxford University Press. p. 486. ISBN 978-0-19-856837-7.
- ^ Fung VS, Thompson PD (2007). "Rigidity and spasticity". In Tolosa E, Jankovic (eds.). Parkinson's disease and movement disorders. Hagerstown, MD: Lippincott Williams & Wilkins. pp. 504–13. ISBN 978-0-7817-7881-7.
- ^ O'Sullivan SB, Schmitz TJ (2007). "Parkinson's Disease". Physical Rehabilitation (5th ed.). Philadelphia: F.A. Davis. pp. 856–7.
- ^ a b Yao, S.C.; Hart, A.D.; Terzella, M.J. (May 2013). "An evidence-based osteopathic approach to Parkinson disease". Osteopathic Family Physician. 5 (3): 96–101. doi:10.1016/j.osfp.2013.01.003.
- ^ Russell JA, Ciucci MR, Connor NP, Schallert T (23 June 2010). "Targeted exercise therapy for voice and swallow in persons with Parkinson's disease". Brain Research. 1341: 3–11. doi:10.1016/j.brainres.2010.03.029. PMC 2908992. PMID 20233583.
- ^ a b c d e Caballol N, Martí MJ, Tolosa E (September 2007). "Cognitive dysfunction and dementia in Parkinson disease". Movement Disorders. 22 (Suppl 17): S358–S366. doi:10.1002/mds.21677. PMID 18175397. S2CID 3229727.
- ^ Murray ED, Buttner EA, Price BH (2012). "Depression and Psychosis in Neurological Practice". In Bradley WG, Daroff RB, Fenichel GM, Jankovic J (eds.). Bradley's Neurology in Clinical Practice: Expert Consult – Online and Print, 6e (Bradley, Neurology in Clinical Practice e-dition 2v Set). Vol. 1 (6th ed.). Philadelphia, PA: Elsevier/Saunders. pp. 102–103. ISBN 978-1-4377-0434-1.
{{cite book}}: Invalid|display-editors=4(help) - ^ a b "Impulse control disorders in Parkinson's disease: definition, epidemiology, risk factors, neurobiology and management". Parkinsonism Relat. Disord. 15 (Suppl 4): S111–5. December 2009. doi:10.1016/S1353-8020(09)70847-8. PMID 20123548.
{{cite journal}}: Unknown parameter|authors=ignored (help) - ^ "A preliminary investigation of laterality in Parkinson's disease and susceptibility to psychosis". J. Neurol. Neurosurg. Psychiatr. 65 (4): 610–1. October 1998. doi:10.1136/jnnp.65.4.610. PMC 2170290. PMID 9771806.
{{cite journal}}: Unknown parameter|authors=ignored (help) - ^ Friedman JH (June 2010). "Parkinson's disease psychosis 2010: A review article". Parkinsonism Relat. Disord. 16 (9): 553–60. doi:10.1016/j.parkreldis.2010.05.004. PMID 20538500.
- ^ a b c d e f g h i j "Major nutritional issues in the management of Parkinson's disease". Mov. Disord. 24 (13): 1881–92. October 2009. doi:10.1002/mds.22705. hdl:2434/67795. PMID 19691125. S2CID 23528416.
{{cite journal}}: Unknown parameter|authors=ignored (help) - ^ a b c d e f g h i j "Parkinson's disease: from monogenic forms to genetic susceptibility factors". Hum. Mol. Genet. 18 (R1): R48–59. April 2009. doi:10.1093/hmg/ddp012. PMID 19297401.
{{cite journal}}: Unknown parameter|authors=ignored (help) - ^ a b c d e f g Davie CA (2008). "A review of Parkinson's disease". Br. Med. Bull. 86 (1): 109–27. doi:10.1093/bmb/ldn013. PMID 18398010.
- ^ IPDGC; Nalls, MA; Plagnol, V; Hernandez, DG; Sharma, M; Sheerin, UM; Saad, M; Simón-Sánchez, J; et al. (2011). "Imputation of sequence variants for identification of genetic risks for Parkinson's disease: a meta-analysis of genome-wide association studies". Lancet. 377 (9766): 641–49. doi:10.1016/S0140-6736(10)62345-8. PMC 3696507. PMID 21292315.
- ^ Gan-Or, Ziv; Dion, Patrick A.; Rouleau, Guy A. (2015-09-02). "Genetic perspective on the role of the autophagy-lysosome pathway in Parkinson disease". Autophagy. 11 (9): 1443–1457. doi:10.1080/15548627.2015.1067364. ISSN 1554-8627. PMC 4590678. PMID 26207393.
- ^ a b c d e f g h "Functional organization of the basal ganglia: therapeutic implications for Parkinson's disease". Mov. Disord. 23 (Suppl 3): S548–59. 2008. doi:10.1002/mds.22062. PMID 18781672. S2CID 13186083.
{{cite journal}}: Unknown parameter|authors=ignored (help) - ^ a b c Dickson DV (2007). "Neuropathology of movement disorders". In Tolosa E, Jankovic JJ (ed.). Parkinson's disease and movement disorders. Hagerstown, MD: Lippincott Williams & Wilkins. pp. 271–83. ISBN 978-0-7817-7881-7.
- ^ Gendelman, Howard E., ed. (2009). "Regional brain stem atrophy in idiopathic Parkinson's disease detected by anatomical MRI". PLOS ONE. 4 (12): e8247. Bibcode:2009PLoSO...4.8247J. doi:10.1371/journal.pone.0008247. PMC 2784293. PMID 20011063.
{{cite journal}}: Unknown parameter|authors=ignored (help) - ^ a b c d e f g h i j k l m n o p q r s "Missing pieces in the Parkinson's disease puzzle". Nat. Med. 16 (6): 653–61. May 2010. doi:10.1038/nm.2165. PMID 20495568. S2CID 3146438.
{{cite journal}}: Unknown parameter|authors=ignored (help) - ^ a b c Schulz-Schaeffer WJ (August 2010). "The synaptic pathology of alpha-synuclein aggregation in dementia with Lewy bodies, Parkinson's disease and Parkinson's disease dementia". Acta Neuropathol. 120 (2): 131–43. doi:10.1007/s00401-010-0711-0. PMC 2892607. PMID 20563819.
- ^ Hirsch EC (December 2009). "Iron transport in Parkinson's disease". Parkinsonism Relat. Disord. 15 (Suppl 3): S209–11. doi:10.1016/S1353-8020(09)70816-8. PMID 20082992.
- ^ a b The National Collaborating Centre for Chronic Conditions, ed. (2006). "Diagnosing Parkinson's Disease". Parkinson's Disease. London: Royal College of Physicians. pp. 29–47. ISBN 1-86016-283-5.
- ^ "The differential diagnosis of Parkinson's disease". Eur. J. Neurol. 9 (Suppl 3): 23–30. November 2002. doi:10.1046/j.1468-1331.9.s3.3.x. PMID 12464118.
{{cite journal}}: Unknown parameter|authors=ignored (help) - ^ Postuma RB, Berg D, Stern M, Poewe W, Olanow CW, Oertel W, Obeso J, Marek K, Litvan I, Lang AE, Halliday G, Goetz CG, Gasser T, Dubois B, Chan P, Bloem BR, Adler CH, Deuschl G. "MDS clinical diagnostic criteria for Parkinson's disease." Mov Disord. 2015 Oct;30(12):1591-601. doi: 10.1002/mds.26424. PMID:26474316
- ^ Berg D, Postuma RB, Adler CH, Bloem BR, Chan P, Dubois B, Gasser T, Goetz CG, Halliday G, Joseph L, Lang AE, Liepelt-Scarfone I, Litvan I, Marek K, Obeso J, Oertel W, Olanow CW, Poewe W, Stern M, Deuschl G. "MDS research criteria for prodromal Parkinson's disease" Mov Disord. 2015 Oct;30(12):1600-11. doi: 10.1002/mds.26431. PMID: 26474317
- ^ a b c d e Brooks DJ (April 2010). "Imaging approaches to Parkinson disease". J. Nucl. Med. 51 (4): 596–609. doi:10.2967/jnumed.108.059998. PMID 20351351. S2CID 17720009.
- ^ Wood, H (June 2014). "Parkinson disease: 18F-DTBZ PET tracks dopaminergic degeneration in patients with Parkinson disease". Nature Reviews. Neurology. 10 (6): 305. doi:10.1038/nrneurol.2014.81. PMID 24840973. S2CID 30591628.
- ^ a b Ahlskog, JE (19 July 2011). "Does vigorous exercise have a neuroprotective effect in Parkinson disease?". Neurology. 77 (3): 288–94. doi:10.1212/wnl.0b013e318225ab66. PMC 3136051. PMID 21768599.
- ^ "Caffeine exposure and the risk of Parkinson's disease: a systematic review and meta-analysis of observational studies". J. Alzheimers Dis. 20 (Suppl 1): S221–38. 2010. doi:10.3233/JAD-2010-091525. PMID 20182023.
{{cite journal}}: Unknown parameter|authors=ignored (help) - ^ a b c d e f g h i j "Epidemiology of Parkinson's disease". Lancet Neurol. 5 (6): 525–35. June 2006. doi:10.1016/S1474-4422(06)70471-9. PMID 16713924. S2CID 39310242.
{{cite journal}}: Unknown parameter|authors=ignored (help) - ^ "Multiple roles for nicotine in Parkinson's disease". Biochem Pharmacol. 78 (7): 677–85. 1 October 2009. doi:10.1016/j.bcp.2009.05.003. PMC 2815339. PMID 19433069.
{{cite journal}}: Unknown parameter|authors=ignored (help) - ^ "Tobacco leaf, smoke and smoking, MAO inhibitors, Parkinson's disease and neuroprotection; are there links?". Neurotoxicology. 25 (1–2): 279–91. January 2004. doi:10.1016/S0161-813X(03)00107-4. PMID 14697903.
{{cite journal}}: Unknown parameter|authors=ignored (help) - ^ Ritz B, Lee PC, Lassen CF, Arah OA. "Parkinson disease and smoking revisited: ease of quitting is an early sign of the disease" Neurology 2014; 83: 1396–402. PMID: 25217056
- ^ a b c d e f g h i j k l m n o p q r s t u v w x y z aa ab ac The National Collaborating Centre for Chronic Conditions, ed. (2006). "Symptomatic pharmacological therapy in Parkinson's disease". Parkinson's Disease. London: Royal College of Physicians. pp. 59–100. ISBN 1-86016-283-5.
- ^ a b Bronstein, M.; et al. (February 2011). "Deep brain stimulation for Parkinson disease: an expert consensus and review of key issues". Arch. Neurol. 68 (2): 165. doi:10.1001/archneurol.2010.260. PMC 4523130. PMID 20937936.
- ^ The National Collaborating Centre for Chronic Conditions, ed. (2006). "Palliative care in Parkinson's disease". Parkinson's Disease. London: Royal College of Physicians. pp. 147–51. ISBN 1-86016-283-5.
- ^ Tolosa E, Jankovic JJ, ed. (2007). "Pharmacological management of Parkinson's disease". Parkinson's disease and movement disorders. Hagerstwon, MD: Lippincott Williams & Wilkins. pp. 110–45. ISBN 978-0-7817-7881-7.
{{cite book}}: Unknown parameter|authors=ignored (help) - ^ Hauser RA, Hsu A, Kell S, Espay AJ, Sethi K, Stacy M, Ondo W, O'Connell M, Gupta S; "IPX066 ADVANCE-PD investigators. Extended-release carbidopa-levodopa (IPX066) compared with immediate-release carbidopa-levodopa in patients with Parkinson's disease and motor fluctuations: a phase 3 randomised, double-blind trial." Lancet Neurol. 2013 Apr;12(4):346-56. doi: 10.1016/S1474-4422(13)70025-5. PMID: 23485610
- ^ Olanow CW, Kieburtz K, Odin P, Espay AJ, Standaert DG, Fernandez HH, Vanagunas A, Othman AA, Widnell KL, Robieson WZ, Pritchett Y, Chatamra K, Benesh J, Lenz RA, Antonini A; LCIG Horizon Study Group. "Continuous intrajejunal infusion of levodopa-carbidopa intestinal gel for patients with advanced Parkinson's disease: a randomised, controlled, double-blind, double-dummy study." Lancet Neurol. 2014 Feb;13(2):141-9. doi: 10.1016/S1474-4422(13)70293-X. PMID: 24361112
- ^ Goldenberg MM (October 2008). "Medical management of Parkinson's disease". P & T. 33 (10): 590–606. PMC 2730785. PMID 19750042.
- ^ PD Med Collaborative Group, Gray R, Ives N, Rick C, Patel S, Gray A, Jenkinson C, McIntosh E, Wheatley K, Williams A, Clarke CE. "Long-term effectiveness of dopamine agonists and monoamine oxidase B inhibitors compared with levodopa as initial treatment for Parkinson's disease (PD MED): a large, open-label, pragmatic randomised trial". Lancet. 2014 Sep 27;384(9949):1196-205. doi: 10.1016/S0140-6736(14)60683-8. PMID: 24928805
- ^ Pondal M, Marras C, Miyasaki J, Moro E, Armstrong MJ, Strafella AP, Shah BB, Fox S, Prashanth LK, Phielipp N, Lang AE. "Clinical features of dopamine agonist withdrawal syndrome in a movement disorders clinic" J Neurol Neurosurg Psychiatry 2013 Feb;84(2):130-5. doi: 10.1136/jnnp-2012-302684 PMID: 22933817
- ^ Thobois S, Lhommée E, Klinger H, Ardouin C, Schmitt E, Bichon A, Kistner A, Castrioto A, Xie J, Fraix V, Pelissier P, Chabardes S, Mertens P, Quesada JL, Bosson JL, Pollak P, Broussolle E, Krack P. "Parkinsonian apathy responds to dopaminergic stimulation of D2/D3 receptors with piribedil." Brain. 2013 May;136(Pt 5):1568-77. doi: 10.1093/brain/awt067. PMID: 23543483
- ^ a b The National Collaborating Centre for Chronic Conditions, ed. (2006). "Non-motor features of Parkinson's disease". Parkinson's Disease. London: Royal College of Physicians. pp. 113–33. ISBN 1-86016-283-5.
- ^ "Pharmacological management of psychosis in elderly patients with parkinsonism". Am. J. Med. 122 (7): 614–22. July 2009. doi:10.1016/j.amjmed.2009.01.025. PMID 19559160.
{{cite journal}}: Unknown parameter|authors=ignored (help) - ^ a b c d The National Collaborating Centre for Chronic Conditions, ed. (2006). "Surgery for Parkinson's disease". Parkinson's Disease. London: Royal College of Physicians. pp. 101–11. ISBN 1-86016-283-5.
- ^ a b c d e The National Collaborating Centre for Chronic Conditions, ed. (2006). "Other key interventions". Parkinson's Disease. London: Royal College of Physicians. pp. 135–46. ISBN 1-86016-283-5.
- ^ a b "The effectiveness of exercise interventions for people with Parkinson's disease: a systematic review and meta-analysis". Mov. Disord. 23 (5): 631–40. April 2008. doi:10.1002/mds.21922. PMID 18181210. S2CID 3808899.
{{cite journal}}: Unknown parameter|authors=ignored (help) - ^ "Comparison of the effects of a physiotherapist-supervised exercise programme and a self-supervised exercise programme on quality of life in patients with Parkinson's disease". Clin Rehabil. 24 (4): 352–62. April 2010. doi:10.1177/0269215509358933. PMID 20360152. S2CID 10947269.
{{cite journal}}: Unknown parameter|authors=ignored (help) - ^ O'Sullivan & Schmitz 2007, pp. 873, 876
- ^ O'Sullivan & Schmitz 2007, p. 879
- ^ O'Sullivan & Schmitz 2007, p. 877
- ^ O'Sullivan & Schmitz 2007, p. 880
- ^ "The science and practice of LSVT/LOUD: neural plasticity-principled approach to treating individuals with Parkinson disease and other neurological disorders". Semin. Speech. Lang. 27 (4): 283–99. November 2006. doi:10.1055/s-2006-955118. PMID 17117354.
{{cite journal}}: Unknown parameter|authors=ignored (help) - ^ Deane, Katherine, ed. (2007). "Occupational therapy for patients with Parkinson's disease". Cochrane Database of Systematic Reviews (3): CD002813. doi:10.1002/14651858.CD002813.pub2. PMC 6991932. PMID 17636709.
{{cite journal}}: Unknown parameter|authors=ignored (help) - ^ "The national agenda for quality palliative care: the National Consensus Project and the National Quality Forum". J Pain Symptom Manage. 33 (6): 737–44. 2007. doi:10.1016/j.jpainsymman.2007.02.024. PMID 17531914.
{{cite journal}}: Unknown parameter|authors=ignored (help) - ^ a b "Palliative treatment of chronic neurologic disorders". Handb Clin Neurol. Handbook of Clinical Neurology. 118: 133–9. 2013. doi:10.1016/B978-0-444-53501-6.00010-X. ISBN 9780444535016. PMID 24182372.
{{cite journal}}: Unknown parameter|authors=ignored (help) - ^ a b Ghoche R (2012). "The conceptual framework of palliative care applied to advanced Parkinson's disease". Parkinsonism Relat Disord. 18 (Suppl 3): S2–5. doi:10.1016/j.parkreldis.2012.06.012. PMID 22771241.
- ^ a b c Wilcox SK (2010). "Extending palliative care to patients with Parkinson's disease". Br J Hosp Med (Lond). 71 (1): 26–30. doi:10.12968/hmed.2010.71.1.45969. PMID 20081638.
- ^ "Are there differences in the prevalence of palliative care-related problems in people living with advanced cancer and eight non-cancer conditions? A systematic review". J Pain Symptom Manage. Online first (4): 660–677. 2014. doi:10.1016/j.jpainsymman.2013.11.009. PMID 24801658.
{{cite journal}}: Unknown parameter|authors=ignored (help) - ^ Casey G (2013). "Parkinson's disease: a long and difficult journey". Nurs N Z. 19 (7): 20–4. PMID 24195263.
- ^ Koch G (2010). "rTMS effects on levodopa induced dyskinesias in Parkinson's disease patients: searching for effective cortical targets". Restor. Neurol. Neurosci. 28 (4): 561–8. doi:10.3233/RNN-2010-0556. PMID 20714078.
- ^ "Brain stimulation and brain repair—rTMS: from animal experiment to clinical trials—what do we know?". Restor. Neurol. Neurosci. 28 (4): 387–98. 2010. doi:10.3233/RNN-2010-0570. PMID 20714064.
{{cite journal}}: Unknown parameter|authors=ignored (help) - ^ "Controlled trial on the effect of 10 days low-frequency repetitive transcranial magnetic stimulation (rTMS) on motor signs in Parkinson's disease". Mov. Disord. 25 (12): 1830–8. September 2010. doi:10.1002/mds.23055. hdl:2183/14505. PMID 20669300. S2CID 2211189.
{{cite journal}}: Unknown parameter|authors=ignored (help) - ^ "Practice Parameter: neuroprotective strategies and alternative therapies for Parkinson disease (an evidence-based review): report of the Quality Standards Subcommittee of the American Academy of Neurology". Neurology. 66 (7): 976–82. April 2006. doi:10.1212/01.wnl.0000206363.57955.1b. PMID 16606908. S2CID 219207854.
{{cite journal}}: Unknown parameter|authors=ignored (help) - ^ "Effectiveness of tai chi for Parkinson's disease: a critical review". Parkinsonism Relat. Disord. 14 (8): 589–94. December 2008. doi:10.1016/j.parkreldis.2008.02.003. PMID 18374620.
{{cite journal}}: Unknown parameter|authors=ignored (help) - ^ "Qigong for movement disorders: A systematic review". Mov. Disord. 24 (2): 301–3. January 2009. doi:10.1002/mds.22275. PMID 18973253. S2CID 206239252.
{{cite journal}}: Unknown parameter|authors=ignored (help) - ^ "Effectiveness of acupuncture for Parkinson's disease: a systematic review". Mov. Disord. 23 (11): 1505–15. August 2008. doi:10.1002/mds.21993. PMID 18618661. S2CID 24713983.
{{cite journal}}: Unknown parameter|authors=ignored (help) - ^ "Mucuna pruriens in Parkinson's disease: a double blind clinical and pharmacological study". J. Neurol. Neurosurg. Psychiatr. 75 (12): 1672–7. 2004. doi:10.1136/jnnp.2003.028761. PMC 1738871. PMID 15548480.
{{cite journal}}: Unknown parameter|authors=ignored (help) - ^ "Case of neuroleptic malignant-like syndrome precipitated by abrupt fava bean discontinuance". Mov. Disord. 20 (5): 630–1. May 2005. doi:10.1002/mds.20380. PMID 15719433. S2CID 37525204.
{{cite journal}}: Unknown parameter|authors=ignored (help) - ^ "Fava beans and Parkinson's disease: useful 'natural supplement' or useless risk?". Eur. J. Neurol. 16 (10): e171. October 2009. doi:10.1111/j.1468-1331.2009.02766.x. PMID 19678834. S2CID 33221249.
{{cite journal}}: Unknown parameter|authors=ignored (help) - ^ a b c d e f g h i j k l Poewe W (December 2006). "The natural history of Parkinson's disease". J. Neurol. 253 (Suppl 7): VII2–6. doi:10.1007/s00415-006-7002-7. PMID 17131223. S2CID 35082340.
- ^ a b GBD 2013 Mortality and Causes of Death, Collaborators (17 December 2014). "Global, regional, and national age-sex specific all-cause and cause-specific mortality for 240 causes of death, 1990–2013: a systematic analysis for the Global Burden of Disease Study 2013". Lancet. 385 (9963): 117–71. doi:10.1016/S0140-6736(14)61682-2. PMC 4340604. PMID 25530442.
{{cite journal}}:|first1=has generic name (help)CS1 maint: numeric names: authors list (link) - ^ a b c García Ruiz PJ (December 2004). "Prehistoria de la enfermedad de Parkinson" [[Prehistory of Parkinson's disease]]. Neurologia (in Spanish). 19 (10): 735–7. PMID 15568171.
- ^ a b Lanska DJ (2010). "Chapter 33: the history of movement disorders". Handb. Clin. Neurol. Handbook of Clinical Neurology. 95: 501–46. doi:10.1016/S0072-9752(08)02133-7. ISBN 9780444520098. PMID 19892136.
- ^ "Tremor in Latin texts of Dutch physicians: 16th–18th centuries". Mov. Disord. 12 (5): 798–806. September 1997. doi:10.1002/mds.870120531. PMID 9380070. S2CID 310819.
{{cite journal}}: Unknown parameter|authors=ignored (help) - ^ a b c d e f g Lees AJ (September 2007). "Unresolved issues relating to the shaking palsy on the celebration of James Parkinson's 250th birthday". Mov. Disord. 22 (Suppl 17): S327–34. doi:10.1002/mds.21684. PMID 18175393. S2CID 9471754.
- ^ Louis ED (November 1997). "The shaking palsy, the first forty-five years: a journey through the British literature". Mov. Disord. 12 (6): 1068–72. doi:10.1002/mds.870120638. PMID 9399240. S2CID 34630080.
- ^ a b c Fahn S (2008). "The history of dopamine and levodopa in the treatment of Parkinson's disease". Mov. Disord. 23 (Suppl 3): S497–508. doi:10.1002/mds.22028. PMID 18781671. S2CID 45572523.
- ^ "A brief history of pallidotomy". Neurosurgery. 41 (5): 1169–80, discussion 1180–3. November 1997. doi:10.1097/00006123-199711000-00029. PMID 9361073.
{{cite journal}}: Unknown parameter|authors=ignored (help) - ^ Hornykiewicz O (2002). "L-DOPA: from a biologically inactive amino acid to a successful therapeutic agent". Amino Acids. 23 (1–3): 65–70. doi:10.1007/s00726-001-0111-9. PMID 12373520. S2CID 25117208.
- ^ a b c d e Findley LJ (September 2007). "The economic impact of Parkinson's disease". Parkinsonism Relat. Disord. 13 (Suppl): S8–S12. doi:10.1016/j.parkreldis.2007.06.003. PMID 17702630.
- ^ a b "Parkinson's – 'the shaking palsy'". GlaxoSmithKline. 1 April 2009.
- ^ "National Parkinson Foundation – Mission". Retrieved 28 March 2011.
- ^ "Education: Joy in Giving". Time. 18 January 1960. Retrieved 2 April 2011.
- ^ "About PDF". Parkinson's Disease Foundation. Retrieved 22 July 2015.
- ^ "American Parkinson Disease Association: Home". American Parkinson Disease Association. Retrieved 9 August 2010.
- ^ "About EPDA". European Parkinson's Disease Association. 2010. Retrieved 9 August 2010.
- ^ Davis P (3 May 2007). "Michael J. Fox". The TIME 100. Time. Retrieved 2 April 2011.
- ^ a b c Brockes E (11 April 2009). "'It's the gift that keeps on taking'". The Guardian. Retrieved 25 October 2010.
- ^ "Michael J. Fox to be made honorary doctor at Karolinska Institutet". Karolinska Institutet. 5 March 2010. Retrieved 2 April 2011.
- ^ Macur, Juliet (26 March 2008). "For the Phinney Family, a Dream and a Challenge". The New York Times. Retrieved 25 May 2013.
About 1.5 million Americans have received a diagnosis of Parkinson's disease, but only 5 to 10 percent learn of it before age 40, according to the National Parkinson Foundation. Davis Phinney was among the few.
- ^ "Who We Are". Davis Phinney Foundation. Retrieved 18 January 2012.
- ^ Emanuel, W (19 October 2010). "Davis Phinney foundation launches exercise-focused tools to help people live well with Parkinson's" (PDF) (Press release). Davis Phinney foundation. Retrieved 2 April 2011.
- ^ Brey RL (April 2006). "Muhammad Ali's Message: Keep Moving Forward". Neurology Now. 2 (2). American Academy of Neurology: 8. doi:10.1097/01222928-200602020-00003. Retrieved 2 April 2011.
- ^ Matthews W (April 2006). "Ali's Fighting Spirit". Neurology Now. 2 (2). American Academy of Neurology: 10–23. doi:10.1097/01222928-200602020-00004. S2CID 181104230. Retrieved 2 April 2011.
- ^ Tauber P (17 July 1988). "Ali: Still Magic". New York Times. Retrieved 2 April 2011.
- ^ a b Dimond PF (16 August 2010). "No New Parkinson Disease Drug Expected Anytime Soon". GEN news highlights. GEN-Genetic Engineering & Biotechnology News.
- ^ Blum, D; Torch, S; Lambeng, N; Nissou, M; Benabid, AL; Sadoul, R; Verna, JM (October 2001). "Molecular pathways involved in the neurotoxicity of 6-OHDA, dopamine and MPTP: contribution to the apoptotic theory in Parkinson's disease". Progress in Neurobiology. 65 (2): 135–172. doi:10.1016/S0301-0082(01)00003-X. PMID 11403877. S2CID 19095092.
- ^ "Chronic Parkinsonism in humans due to a product of meperidine-analog synthesis". Science. 219 (4587): 979–80. February 1983. Bibcode:1983Sci...219..979L. doi:10.1126/science.6823561. PMID 6823561.
{{cite journal}}: Unknown parameter|authors=ignored (help) - ^ "Environmental toxins and Parkinson's disease: what have we learned from pesticide-induced animal models?". Trends Pharmacol. Sci. 30 (9): 475–83. September 2009. doi:10.1016/j.tips.2009.06.005. PMID 19729209.
{{cite journal}}: Unknown parameter|authors=ignored (help) - ^ "Transgenic rodent models of Parkinson's disease". Acta Neurochir. Suppl. Acta Neurochirurgica Supplementum. 101: 89–92. 2008. doi:10.1007/978-3-211-78205-7_15. ISBN 978-3-211-78204-0. PMC 2613245. PMID 18642640.
{{cite journal}}: Unknown parameter|authors=ignored (help) - ^ "Gene Therapy in Parkinson's Disease: Rationale and Current Status". CNS Drugs. 24 (3): 177–92. 2010. doi:10.2165/11533740-000000000-00000. PMC 2886503. PMID 20155994.
{{cite journal}}: Unknown parameter|authors=ignored (help) - ^ "AAV2-GAD gene therapy for advanced Parkinson's disease: a double-blind, sham-surgery controlled, randomised trial". Lancet Neurol. 10 (4): 309–19. April 2011. doi:10.1016/S1474-4422(11)70039-4. PMID 21419704. S2CID 37154043.
{{cite journal}}: Unknown parameter|authors=ignored (help) - ^ "Neurologix Files to Liquidate Under Chapter 7 Bankruptcy". Bloomberg News.
- ^ Warren Olanow C, Bartus RT, Baumann TL, Factor S, Boulis N, Stacy M, Turner DA, Marks W, Larson P, Starr PA, Jankovic J, Simpson R, Watts R, Guthrie B, Poston K, Henderson JM, Stern M, Baltuch G, Goetz CG, Herzog C, Kordower JH, Alterman R, Lozano AM, Lang AE. "Gene delivery of neurturin to putamen and substantia nigra in Parkinson disease: A double-blind, randomized, controlled trial." Ann Neurol. 2015 Aug;78(2):248-57. doi: 10.1002/ana.24436. PMID: 26061140
- ^ "World's first Parkinson's vaccine is trialled". New Scientist. 7 June 2012.
- ^ Redmond DE (October 2002). "Cellular replacement therapy for Parkinson's disease—where we are today?". The Neuroscientist. 8 (5): 457–88. doi:10.1177/107385802237703. PMID 12374430.
- ^ "Stem Cell Research Aims to Tackle Parkinson's Disease". Retrieved 16 April 2010.
- ^ Olanow CW, Kordower JH, Lang AE, Obeso JA. "Dopaminergic transplantation for Parkinson's disease: current status and future prospects." Ann Neurol. 2009 Nov;66(5):591-6. doi: 10.1002/ana.21778. PMID: 19938101
