User:Anonchemist/sandbox
Roy:
Inorganic synthesis
[edit]Inorganic synthesis and organometallic synthesis are used to prepare compounds with significant non-organic content. An illustrative example is the preparation of the anti-cancer drug cisplatin from potassium tetrachloroplatinate.[1]
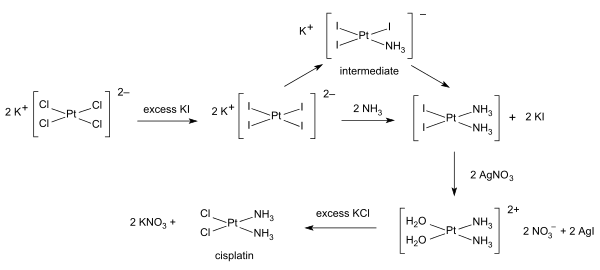
Inorganic Steps
[edit]Inorganic synthesis can be incredibly complex, and involve several intermediates and elementary steps. The steps outlined below are a few of the types of steps that can occur during an inorganic synthesis.
Oxidative Additiion
[edit]Oxidative Addition involves a metal center and a molecule with at least two atoms; the atoms can be of the same species, but do not necessarily have to be. The bond between the two atoms will break, and 2 new bonds will form between those atoms and the metal center. [2]

This is reffered to as "oxidative" because atoms A and B oxidize the metal; that is, the oxidation state of the metal is +2 relative to the oxidation state of the metal before the oxidative addition took place.[2]
Reductive Elimination
[edit]Reductive Elimination is the reverse reaction of Oxidative Addition. It invovles two atoms, A and B, bonded to a metal center. Reductive elimination will see atoms A and B form a bond with each other while both losing their bonds with the metal center.[3]
This is reffered to as "Reductive" because this reaction reduces the metla center; that is, the metal center's oxidation state will be 2 lower than it was before the reaction took place. [4]
Ligand Substitution
[edit]Ligand Substitution occurs when a chemical species attached the a metal center is replaced with a different one.
Associative Substitution
[edit]
Associative Substitution sees an incoming ligand Y coordinate to the metal center as the first step. Only after the association is complete, will the leaving ligand X leave the metal center, completing the substution process.[5]
This mechanism tends to occur with complexes that are unsaturated in both ligands and electrons; that is, ligands with <6 ligands and <18 valence electrons.[6]
The first step is generally rate determining, meaning that the reaction rate is second order; the reaction speed dependsd on both the concentration of the metal center and the concentration of the [Y] species.[7]
Dissociative Substitution
[edit]Dissociative substution sees the outgoing ligand X leave the metal center before the incoming lingad Y coordinates with the metal center. Only after X completely leaves does the new ligand Y coordinate to the metal center.
This mechanism tends to occur with ligands that are fully staurated; that is, complexes with 6 ligands and 18 valence electrons.
The rate determing step tends to be the dissociation step. Thus, the rate tends to be first order; the reaction rate depends on the concentration of the metal center, and is independant of both the concentration and identity of ligand Y.
Susan:
Organic Synthesis
[edit]SN1 Reaction:
[edit]SN1 is a class of nucleophilic substitution reactions where it involves 2 steps between a central carbon and leaving group. The first step is a slow, rate determining step where the bond between the central atom and leaving group breaks forming a carbocation.[8] The second step is faster, as it involves a nucleophile attacking the empty carbocation. It removes the leaving group in the molecule and forms a new C-Nu bond. SN1 reactions are best reacted with secondary or tertiary alkyl halides, with tertiary alkyl halides being the most optimal molecule to react with.

SN2 Reaction:
[edit]Similar to SN1, SN2 is also a class of nucleophilic substitution reactions where it involves 2 steps between a central carbon and leaving group. SN2 reactions are concerted, meaning they are a one step process.[9] It is a process where the nucleophile attacks an electrophilic carbon, and the bond of leaving group and carbon will be broken at the same time. A set of lone pair electrons from the nucleophile attacks the electrophilic carbon of the alkyl halide to form a sp2 transition state, then forms a new C-Nu bond. SN2 reactions are best reacted with primary or secondary alkyl halides, with primary alkyl halides being the most optimal molecule to react with.

Important Applications in Organic Synthesis:
[edit]Suzuki-Miyaura Cross-Coupling:
[edit]**add link for wiki article
Suzuki-Miyaura Cross-Coupling is a metal-catalyzed reaction involving a palladium catalyst to cross-couple a boronic acid to an organohalide.[10][11] The reaction is used to create new carbon-carbon bonds to make conjugated systems of compounds such as alkenes, styrenes and biaryl compounds.[12] Suzuki coupling involves 3 steps: oxidative addition, transmetallation and reductive elimination.

There are important applications in Suzuki-Miyaura Cross Coupling, as their advantages are that they can react in mild reaction conditions and their starting materials are easy to obtain. They are also known for their low toxicity, as boronic acids are easy to handle and obtain in the market. This reaction is commonly used for synthesizing biaryls, which are important compounds in industrial chemistry and pharmaceuticals.[13] Medical fields utilize biaryls as a key step producing bio-active molecules for medicines. They are privileged structures that have a high affinity and are capable of binding to multiple receptors for biological activity.[14] Other applications include producing polymers for electronic chip insulation and using compounds for organic light emitting diodes. Suzuki-Miyaura Cross-Coupling has important applications for polymer synthesis.
Polymer Synthesis:
[edit]Polymers are long-chain repeating molecules that are created from monomers, which are joined together by covalent bonding to form polymer structures. Polymer synthesis has important applications in materials chemistry and medical fields, as they are essential for creating nano materials in medicine which helps diagnose, treat, and prevents certain types of diseases. There are two groups of polymer synthesis reactions: condensation and addition polymerization.
In condensation polymerization, it involves the elimination of small molecules (often water) to form polymers. It happens between two different bi-functional or tri-functional monomers. In addition polymerization, polymer growth requires an initiator that produces the initiator species with reactive centres, such as a free radical, anion, cation, or organometallic complex.
To insert citation:
https://www.sigmaaldrich.com/CA/en/applications/materials-science-and-engineering/polymer-synthesis?srsltid=AfmBOoodcYXkfXwRx5PcF1kmLCkZl4nYxaf6sa3D0QZBk0pE1owmiWoxhttps://www.researchgate.net/publication/367536589_New_Technologies_in_Polymer_Synthesis_and_Applications_of_Polymers#:~:text=Polymer%20nanocomposites%20offer%20to%20modern,applications%2C%20drug%20delivery%2C%20etc.
References
- ^ Alderden, Rebecca A.; Hall, Matthew D.; Hambley, Trevor W. (1 May 2006). "The Discovery and Development of Cisplatin". J. Chem. Educ. 83 (5): 728. Bibcode:2006JChEd..83..728A. doi:10.1021/ed083p728.
- ^ a b Housecroft, Catherine E.; Sharpe, Alan G. (2018). Inorganic chemistry (Fifth edition ed.). Harlow, England London New York Boston San Francisco Toronto Sydney: Pearson. ISBN 978-1-292-13414-7.
{{cite book}}:|edition=has extra text (help) - ^ "Reductive elimination", Wikipedia, 2023-02-12, retrieved 2024-11-05
- ^ "Reductive elimination", Wikipedia, 2023-02-12, retrieved 2024-11-05
- ^ "Associative substitution", Wikipedia, 2022-03-08, retrieved 2024-11-05
- ^ "Associative substitution", Wikipedia, 2022-03-08, retrieved 2024-11-05
- ^ "Associative substitution", Wikipedia, 2022-03-08, retrieved 2024-11-05
- ^ Ashenhurst, James (2012-07-13). "The SN1 Mechanism". Master Organic Chemistry. Retrieved 2024-11-03.
- ^ "11.2: The SN2 Reaction". Chemistry LibreTexts. 2015-05-03. Retrieved 2024-11-03.
- ^ "Suzuki reaction", Wikipedia, 2024-09-26, retrieved 2024-11-03
- ^ Miyaura, Norio; Yamada, Kinji; Suzuki, Akira (1979-01-01). "A new stereospecific cross-coupling by the palladium-catalyzed reaction of 1-alkenylboranes with 1-alkenyl or 1-alkynyl halides". Tetrahedron Letters. 20 (36): 3437–3440. doi:10.1016/S0040-4039(01)95429-2. ISSN 0040-4039.
- ^ Chemler, Sherry R.; Trauner, Dirk; Danishefsky, Samuel J. (2001-12-17). "The B-Alkyl Suzuki-Miyaura Cross-Coupling Reaction: Development, Mechanistic Study, and Applications in Natural Product Synthesis A list of abbreviations can be found at the end of the article". Angewandte Chemie (International Ed. in English). 40 (24): 4544–4568. doi:10.1002/1521-3773(20011217)40:24<4544::aid-anie4544>3.0.co;2-n. ISSN 1521-3773. PMID 12404358.
- ^ "Suzuki-Miyaura Cross-Coupling Reaction". News-Medical. 2018-01-12. Retrieved 2024-11-03.
- ^ "Suzuki-Miyaura Cross-Coupling Reaction". News-Medical. 2018-01-12. Retrieved 2024-11-03.
