User:Aflahert/Isotopic signature
| This is the sandbox page where you will draft your initial Wikipedia contribution.
If you're starting a new article, you can develop it here until it's ready to go live. If you're working on improvements to an existing article, copy only one section at a time of the article to this sandbox to work on, and be sure to use an edit summary linking to the article you copied from. Do not copy over the entire article. You can find additional instructions here. Remember to save your work regularly using the "Publish page" button. (It just means 'save'; it will still be in the sandbox.) You can add bold formatting to your additions to differentiate them from existing content. |
Stable Isotopes
[edit]Sulfur Isotopes
[edit]Sulfur has four stable isotopes, 32S, 33S, 34S, and 36S, of which 32S is the most abundant by far due to the fact it is created by the very common 12C in supernovas. Sulfur isotope ratios are almost always expressed as ratios relative to 32S due to this major relative abundance (95.0%). Sulfur isotope fractionations are usually measured in terms of δ34S due to its higher abundance (4.25%) compared to the other stable isotopes of sulfur, though δ33S is also sometimes measured. Differences in sulfur isotope ratios are thought to exist primarily due to kinetic fractionation during reactions and transformations.
Sulfur isotopes are generally measured against standards; prior to 1993, the Canyon Diablo troilite standard (abbreviated to CDT), which has a 32S:34S equal to 22.220, was used as both a reference material and the zero point for the isotopic scale. Since 1993, the Vienna-CDT standard has been used as a zero point, and there are several materials used as reference materials for sulfur isotope measurements. Sulfur fractionations by natural processes measured against these standards have been shown to exist between -72‰ and +147‰.[1][2]
| Natural Source | δ34S range |
|---|---|
| Petroleum[3] | -32‰ to -8‰ |
| River water[4] | -8‰ to 10‰ |
| Lunar rocks[4] | -2‰ to 2.5‰ |
| Meteorites[4] | 0‰ to 2‰ |
| Ocean water[4] | 17‰ to 20‰ |
| Isotope | Abundance | Half-life |
|---|---|---|
| 32S | 94.99% | Stable |
| 33S | 0.75% | Stable |
| 34S | 4.25% | Stable |
| 35S | <0.1% | 87.4 days |
| 36S | 0.01% | Stable |
As a very redox-active element, sulfur can be useful for recording major chemistry-altering events throughout Earth's history, such as marine evaporites which reflect the change in the atmosphere's redox state brought about by the Oxygen Crisis.[5][6]
Applications
[edit]Records of Early Life on Earth
[edit]Isotope biogeochemistry has been used to investigate the timeline surrounding life and its earliest iterations on Earth. Isotopic fingerprints typical of life, preserved in sediments, have been used to suggest, but do not necessarily prove, that life was already in existence on Earth by 3.85 billion years ago.[7]
Sulfur isotope evidence has also been used to corroborate the timing of the Great Oxidation Event, during which the Earth's atmosphere experienced a measurable rise in oxygen (to about 9% of modern values[8]) for the first time about 2.3-2.4 billion years ago. Because the δ34S values of sulfide minerals is largely controlled by the presence of sulfate-reducing bacteria,[9] the absence of sulfur isotope fractionations in sulfide minerals can indicate the absence of these bacterial processes or the absence of freely available sulfate. (Incomplete--will refine these thoughts and how they connect to the following ones). Mass-independent sulfur isotope fractionations are found to be widespread in the geologic record before about 2.45 billion years ago, and these isotopic signatures have since ceded to mass-dependent fractionation, providing strong evidence that the atmosphere shifted from anoxic to oxygenated at that threshold.[10] (will expand this section briefly).
Modern sulfate-reducing bacteria are known to favorably reduce lighter 32S instead of 34S, and the presence of these microorganisms can measurably alter the sulfur isotope composition of the ocean.[5] Some have used this knowledge of microbial sulfur fractionation to suggest that minerals (namely pyrite) with large sulfur isotope fractionations relative to the inferred seawater composition may be evidence of life.[11][12] This is still sometimes contested, as with the ~3.49 Ga sulfide minerals found in the Dresser Formation of Western Australia, which was found to have δ34S values as large as -22‰.[13] Because it has not been proven that the sulfide and barite minerals formed in the absence of major hydrothermal input, it is not conclusive evidence of life or of the microbial sulfate reduction pathway in the Archean.[14]
(To be Included in the Sulfur Biogeochemistry Article)
[edit]Processes that fractionate sulfur isotopes
[edit]Biological processes that assimilate sulfur
[edit]All organisms metabolize sulfur, and it is incorporated into the structure of proteins, polysaccharides, steroids, and many coenzymes.[15] The biological pathway by which an organism intakes and/or removes sulfur can have significant impacts on the sulfur isotope composition of the organism and its environment.

Some organisms take in relatively small amounts of sulfate in a process called assimilatory sulfate reduction, for the purpose of synthesizing compounds that contain sulfur, such as the amino acids methionine and cysteine that can then be used to make proteins.[17] Literature on the isotopic fractionation effects of the assimilatory sulfate reduction pathway are noticeably less robust than those discussing other microbial sulfur pathways, but some sources believe there to be little to no isotope effects (-4.4‰ < δ34S < +0.5‰).[18]
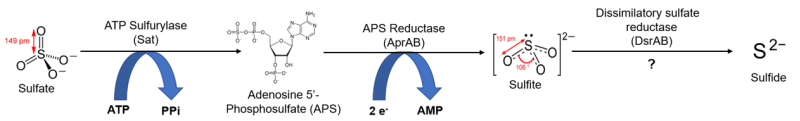
There is also another common pathway by which organisms intake sulfur. These microorganisms, which consume and reduce sulfate in relatively large quantities, are said to perform dissimilatory sulfate reduction. These organisms use sulfate reduction as an energy source as opposed to a way to synthesize new cell components, and remove the resulting sulfide as a waste product. Microbial sulfate reduction has been demonstrated to fractionate sulfur isotopes in bacteria, with some studies showing a dependence upon sulfate concentration[19] and/or temperature[20]
While dissimilatory sulfate reduction and assimilatory sulfate reduction are two of the most common pathways by which organisms uptake and utilize sulfate, there are many other pathways by which living things intake sulfur. For example, sulfur oxidation of compounds like hydrogen sulfide and elemental sulfur is performed by lithotrophic bacteria and chemosynthetic archaea.[21][22] Most animals obtain sulfur directly from the methionine and cysteine in the protein they consume.[23]
References
[edit]- ^ Lever, Mark A.; Rouxel, Olivier; Alt, Jeffrey C.; Shimizu, Nobumichi; Ono, Shuhei; Coggon, Rosalind M.; Shanks, Wayne C.; Lapham, Laura; Elvert, Marcus; Prieto-Mollar, Xavier; Hinrichs, Kai-Uwe (2013-03-01). "Evidence for Microbial Carbon and Sulfur Cycling in Deeply Buried Ridge Flank Basalt". Science. 339 (6125): 1305–1308. doi:10.1126/science.1229240. ISSN 0036-8075.
- ^ Drake, Henrik; Roberts, Nick M. W.; Reinhardt, Manuel; Whitehouse, Martin; Ivarsson, Magnus; Karlsson, Andreas; Kooijman, Ellen; Kielman-Schmitt, Melanie (2021-06-03). "Biosignatures of ancient microbial life are present across the igneous crust of the Fennoscandian shield". Communications Earth & Environment. 2 (1): 1–13. doi:10.1038/s43247-021-00170-2. ISSN 2662-4435.
- ^ Hannan, Keith (1998), "Sulfur isotopes in geochemistry", Geochemistry, Dordrecht: Springer Netherlands, pp. 610–615, doi:10.1007/1-4020-4496-8_309, ISBN 978-1-4020-4496-0, retrieved 2022-05-08
- ^ a b c d "Stable Isotope Geochemistry | SpringerLink" (PDF). doi:10.1007/978-3-030-77692-3.pdf.
{{cite journal}}: Cite journal requires|journal=(help) - ^ a b Seal, Robert R., II (2006-01-01). "Sulfur Isotope Geochemistry of Sulfide Minerals". Reviews in Mineralogy and Geochemistry. 61 (1): 633–677. doi:10.2138/rmg.2006.61.12. ISSN 1529-6466.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ Farquhar, James; Bao, Huiming; Thiemens, Mark (2000-08-04). "Atmospheric Influence of Earth's Earliest Sulfur Cycle". Science. 289 (5480): 756–758. doi:10.1126/science.289.5480.756. ISSN 0036-8075.
- ^ Mojzsis, S. J.; Arrhenius, G.; McKeegan, K. D.; Harrison, T. M.; Nutman, A. P.; Friend, C. R. L. (1996-11). "Evidence for life on Earth before 3,800 million years ago". Nature. 384 (6604): 55–59. doi:10.1038/384055a0. ISSN 1476-4687.
{{cite journal}}: Check date values in:|date=(help) - ^ Holland, Heinrich D (2006-06-29). "The oxygenation of the atmosphere and oceans". Philosophical Transactions of the Royal Society B: Biological Sciences. 361 (1470): 903–915. doi:10.1098/rstb.2006.1838. PMC 1578726. PMID 16754606.
{{cite journal}}: CS1 maint: PMC format (link) - ^ Canfield, D. E. (2001-01-01). "Biogeochemistry of Sulfur Isotopes". Reviews in Mineralogy and Geochemistry. 43 (1): 607–636. doi:10.2138/gsrmg.43.1.607. ISSN 1529-6466.
- ^ Papineau, Dominic; Mojzsis, Stephen J.; Schmitt, Axel K. (2007-03-15). "Multiple sulfur isotopes from Paleoproterozoic Huronian interglacial sediments and the rise of atmospheric oxygen". Earth and Planetary Science Letters. 255 (1): 188–212. doi:10.1016/j.epsl.2006.12.015. ISSN 0012-821X.
- ^ Archer, Corey; Vance, Derek (2006-03-01). "Coupled Fe and S isotope evidence for Archean microbial Fe(III) and sulfate reduction". Geology. 34 (3): 153–156. doi:10.1130/G22067.1. ISSN 0091-7613.
- ^ Wacey, David; McLoughlin, Nicola; Whitehouse, Martin J.; Kilburn, Matt R. (2010-12-01). "Two coexisting sulfur metabolisms in a ca. 3400 Ma sandstone". Geology. 38 (12): 1115–1118. doi:10.1130/G31329.1. ISSN 0091-7613.
- ^ Philippot, Pascal; Zuilen, Mark; Lepot, Kevin; Thomazo, Christophe; Farquhar, James; Van Kranendonk, Martin (2007-09-14). "Early Archaean Microorganisms Preferred Elemental Sulfur, Not Sulfate". Science (New York, N.Y.). 317: 1534–1537. doi:10.1126/science.1145861.
- ^ "Early Life on Earth | SpringerLink" (PDF). doi:10.1007/978-1-4020-9389-0.pdf.
{{cite journal}}: Cite journal requires|journal=(help) - ^ Elliott, Katherine; Whelan, Julie (2009-09-16). Sulphur in Biology. John Wiley & Sons. ISBN 978-0-470-71823-0.
- ^ a b Santos, André A.; Venceslau, Sofia S.; Grein, Fabian; Leavitt, William D.; Dahl, Christiane; Johnston, David T.; Pereira, Inês A. C. (2015-12-18). "A protein trisulfide couples dissimilatory sulfate reduction to energy conservation". Science. 350 (6267): 1541–1545. doi:10.1126/science.aad3558. ISSN 0036-8075.
- ^ "5.9C: Sulfate and Sulfur Reduction". Biology LibreTexts. 2017-05-09. Retrieved 2022-04-27.
- ^ Chambers, L. A.; Trudinger, P. A. (1979-01). "Microbiological fractionation of stable sulfur isotopes: A review and critique". Geomicrobiology Journal. 1 (3): 249–293. doi:10.1080/01490457909377735. ISSN 0149-0451.
{{cite journal}}: Check date values in:|date=(help) - ^ Bradley, A. S.; Leavitt, W.; Schmidt, M.; Knoll, Andrew Herbert; Girguis, Peter R.; Johnston, David T. (2015). "Patterns of sulfur isotope fractionation during microbial sulfate reduction". Geobiology. doi:10.1111/gbi.12149. ISSN 1472-4677.
- ^ Canfield, Donald E.; Olesen, Claus A.; Cox, Raymond P. (2006-02-01). "Temperature and its control of isotope fractionation by a sulfate-reducing bacterium". Geochimica et Cosmochimica Acta. 70 (3): 548–561. doi:10.1016/j.gca.2005.10.028. ISSN 0016-7037.
- ^ Ghosh, Wriddhiman; Dam, Bomba (2009-11-01). "Biochemistry and molecular biology of lithotrophic sulfur oxidation by taxonomically and ecologically diverse bacteria and archaea". FEMS Microbiology Reviews. 33 (6): 999–1043. doi:10.1111/j.1574-6976.2009.00187.x. ISSN 0168-6445.
- ^ Paredes, Gabriela F.; Viehboeck, Tobias; Lee, Raymond; Palatinszky, Marton; Mausz, Michaela A.; Reipert, Siegfried; Schintlmeister, Arno; Maier, Andreas; Volland, Jean-Marie; Hirschfeld, Claudia; Wagner, Michael (2021-06-29). Bordenstein, Seth (ed.). "Anaerobic Sulfur Oxidation Underlies Adaptation of a Chemosynthetic Symbiont to Oxic-Anoxic Interfaces". mSystems. 6 (3): e01186–20. doi:10.1128/mSystems.01186-20. ISSN 2379-5077. PMC 8269255. PMID 34058098.
{{cite journal}}: CS1 maint: PMC format (link) - ^ Komarnisky, Lioudmila A; Christopherson, Robert J; Basu, Tapan K (2003-01-01). "Sulfur: its clinical and toxicologic aspects". Nutrition. 19 (1): 54–61. doi:10.1016/S0899-9007(02)00833-X. ISSN 0899-9007.
