User:Adwctamia/orgo/1
In aqueous solution, ammonia deprotonates a small fraction of the water to give ammonium and hydroxide according to the following equilibrium:
- NH3 + H2O ⇌ NH4+ + OH−.
In a 1M ammonia solution, about 1.42% of the ammonia is converted to ammonium, equivalent to a pH of 11.63. The base ionization constant is
- Kb = [NH4+][OH−]/[NH3] = 1.8×10−5
orgo forums copypasta
[edit]https://en.wikipedia.org/wiki/Simple_aromatic_ring#Heterocyclic_aromatic_rings
When compounds contain more than one functional group, the order of precedence determines which groups are named with prefix or suffix forms. The highest-precedence group takes the suffix, with all others taking the prefix form. However, double and triple bonds only take suffix form (-en and -yn) and are used with other suffixes.
Prefixed substituents are ordered alphabetically (excluding any modifiers such as di-, tri-, etc.), e.g. chlorofluoromethane, not fluorochloromethane. If there are multiple functional groups of the same type, either prefixed or suffixed, the position numbers are ordered numerically (thus ethane-1,2-diol, not ethane-2,1-diol.) The N position indicator for amines and amides comes before "1", e.g. CH3CH(CH3)CH2NH(CH3) is N,2-dimethylpropanamine.
| Priority | Functional group | Formula | Prefix | Suffix |
|---|---|---|---|---|
| 1 | Cations e.g. Ammonium |
NH4+ |
-onio- ammonio- |
-onium -ammonium |
| 2 | Carboxylic acids | –COOH –COSH –COSeH –SO3H –SO2H |
carboxy- sulfanylcarbonyl- selanylcarbonyl- sulfo- sulfino- |
-oic acid* -thioic S-acid* -selenoic Se-acid* -sulfonic acid -sulfinic acid |
| 3 | Carboxylic acid derivatives | –COOR –COX –CONH2 –CON=C< –C(=NH)NH2 |
R-oxycarbonyl- halocarbonyl- carbamoyl- -imido- amidino- |
-R-oate -oyl halide* -amide* -imide* -amidine* |
| 4 | Nitriles Isocyanides |
–CN –NC |
cyano- isocyano- |
-nitrile* isocyanide |
| 5 | Aldehydes Thioaldehydes |
–CHO –CHS |
formyl- thioformyl- |
-al* -thial* |
| 6 | Ketones | =O =S =Se =Te |
oxo- sulfanylidene- selanylidene- tellanylidene- |
-one -thione -selone -tellone |
| 7 | Alcohols | –OH –SH –SeH –TeH |
hydroxy- sulfanyl- selanyl- tellanyl- |
-ol -thiol -selenol -tellurol |
| 8 | Hydroperoxides | -OOH -SOH -SSH |
hydroperoxy- hydroxysulfanyl- disulfanyl- |
-peroxol -SO-thioperoxol -dithioperoxol |
| 9 | Amines Imines Hydrazines |
–NH2 =NH –NHNH2 |
amino- imino- hydrazino- |
-amine -imine -hydrazine |
*Note: These suffixes, in which the carbon atom is counted as part of the preceding chain, are the most commonly used. See individual functional group articles for more details.
The order of remaining functional groups is only needed for substituted benzene and hence is not mentioned here.
Benzannulation
[edit]The term benzannulated compounds refers to derivatives of cyclic compounds (usually aromatic) which are fused to a benzene ring. Examples are listed in the table below:
| Benzannulated derivative | Source of cyclic compound |
|---|---|
| Benzopyrene | Pyrene |
| Quinoline | Pyridine |
| Isoquinoline | |
| Chromene | Pyran |
| Isochromene | |
| Indole | Pyrrole |
| Isoindole | |
| Benzofuran | Furan |
| Isobenzofuran | |
| Benzimidazole | Imidazole |
done
[edit]Nitrogen drama molecules
[edit]Compound classes
[edit]- Nitrogen only
Phenylamine = aniline
Ketones
[edit]Common names for ketones can be derived by naming the two alkyl or aryl groups bonded to the carbonyl group as separate words followed by the word ketone.
| |||
| |||
| Names | |||
|---|---|---|---|
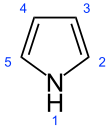
| Preferred IUPAC name
1H-Pyrrole | |||
| Other names
Azole
| |||
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
| |||




ImageFile = Styrene.svg | ImageSize = 150px | ImageFile1 = Styrene-from-xtal-2001-3D-balls.png | ImageSize1 = 180px | ImageName = Styrene]]
new pasta
[edit]An adduct (from the Latin adductus, "drawn toward" alternatively, a contraction of "addition product") is a product of a direct addition of two or more distinct molecules, resulting in a single reaction product containing all atoms of all components.[1] The resultant is considered a distinct molecular species. Examples include the adduct between hydrogen peroxide and sodium carbonate to give sodium percarbonate, and the addition of sodium bisulfite to an aldehyde to give a sulfonate. It can just be considered as a single product resulting from direct addition of different molecules and constitutes all the reactant molecules' atoms. Adducts often form between Lewis acids and Lewis bases.[2] A good example is the formation of adducts between the Lewis acid borane and the oxygen atom in the Lewis bases, tetrahydrofuran (THF): BH3•O(CH2)4 or diethyl ether: BH3•O(CH3CH2)2.