Tris(dimethylamino)methane

| |

| |

| |
| Names | |
|---|---|
| Preferred IUPAC name
N,N,N′,N′,N′′,N′′-Hexamethylmethanetriamine | |
| Other names
N,N,N,N,N,N-hexamethylmethanetriamine
[bis(dimethylamino)methyl]dimethylamine | |
| Identifiers | |
3D model (JSmol)
|
|
| ECHA InfoCard | 100.024.804 |
PubChem CID
|
|
| UNII | |
CompTox Dashboard (EPA)
|
|
| |
| |
| Properties | |
| C7H19N3 | |
| Molar mass | 145.250 g·mol−1 |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
| |
Tris(dimethylamino)methane (TDAM) is the simplest representative of the tris(dialkylamino)methanes of the general formula (R2N)3CH in which three of the four of methane's hydrogen atoms are replaced by dimethylamino groups (−N(CH3)2).[1] Tris(dimethylamino)methane can be regarded as both an amine and an orthoamide.
Tris(dimethylamino)methane is a strong base and can be used as a formylation agent, as aminomethylenation reagent and as a source for the basic bis(dimethylamino)carbene of the formula (R2N)2C:.[2]
Preparation
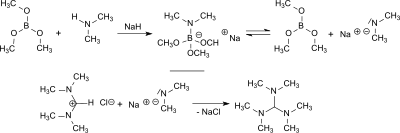
[edit]Tris(dimethylamino)methane is formed in the reaction of N,N,N′,N′-Tetramethylformamidinium chloride (TMF-Cl)[1][3][4] or bis(dimethylamino)acetonitrile[5] with lithium dimethylamide or sodium dimethylamide with yields between 55 and 84%.[2]
From dimethylamine and trimethoxyborane sodium dimethylamide is formed in situ in the presence of sodium hydride which reacts with N,N,N′,N′-tetramethylformamidinium chloride in 84% yield to tris(dimethylamino)methane and with bis(dimethylamino)acetonitrile in 77% yield.[6]
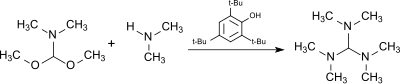
The reaction of the dimethylformamide (DMF) dimethylacetal, HC(OCH3)2N(CH3)2, (from the DMF–dimethyl sulfate complex and sodium methoxide[7]) with dimethylamine in the presence of the acidic catalyst 2,4,6-tri-tert-butylphenol (which is largely stable to the alkylating agent) produces tris(dimethylamino)methane.[8]
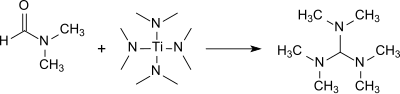
Tris(dimethylamino)methane is formed in good yield (83%) in the reaction of DMF with tetrakis(dimethylamino)titanium(IV).[9]
N,N,N′,N′,N″,N″-Hexamethylguanidinium chloride (readily obtainable by dimethylamine and N,N,N′,N′-tetramethylchloroformamidinium chloride derived from tetramethylurea and phosgene[10]) forms tris(dimethylamino)methane in 53% yield under the exposure of the reducing agent sodium bis(2-methoxyethoxy)aluminium hydride (Red-Al).[11]
Sodium hydride and trimethyl borate reduce N,N,N′,N′,N″,N″-hexamethylguanidinium chloride in 80% yield to tris(dimethylamino)methane.[6]
Properties
[edit]Tris(dimethylamino)methane is a clear, colorless or pale yellow liquid with a strong ammoniacal odor. The compound is freely miscible with many non-polar aprotic and water-free solvents. However, when heated tris(dimethylamino)methane reacts with protic solvents (such as water or alcohols) but also with weak CH-acidic substances, such as acetone or acetonitrile.[2]
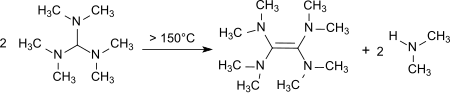
Upon heating to 150–190 °C decomposition occurs with the formation of tetrakis(dimethylamino)ethene,[12] a strong electron donor.[13]
Applications
[edit]Tris(dimethylamino)methane dissociates into N,N,N′,N′-tetramethylformamidinium cations and dimethylamide anions, which abstract protons from CH- and NH-acidic compounds. The anions thus formed add to the formamidinium cations which in turn eliminate dimethylamine and react to form dimethylaminomethylene compounds (= CH−N(CH3)2) or amidines by aminomethyleneation.[1]
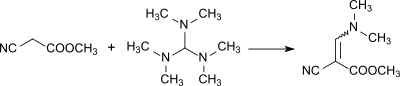
Reaction to form a methyl α-cyano-β-dimethylaminoacrylate:
Reaction to form N,N-dimethyl-N′-p-nitrophenylformamidine:
Aminomethylenation provides intermediates for the synthesis of heterocycles such as pyrimidines, pyrazoles, 1,4-dihydropyridines and indoles.
N,N,N′,N′-Tetramethylselenourea is accessible by the extended heating of tris(dimethylamino)methane with selenium in xylene, bis(dimethylamino)carbene is suggested as an intermediate.[14]
Related reagent
[edit]References
[edit]- ^ a b c Bredereck, H.; Effenberger, F.; Brendle, T. (1966). "Synthese und Reaktionen von Trisdimethylaminomethan" [Synthesis and reaction of tris(dimethylamino)methane]. Angewandte Chemie (in German). 78 (2): 147–148. Bibcode:1966AngCh..78..147B. doi:10.1002/ange.19660780212.
- ^ a b c Kantlehner, W. (2001). "Tris(dimethylamino)methane". Encyclopedia of Reagents for Organic Synthesis. doi:10.1002/047084289X.rt403. ISBN 0-471-93623-5.
- ^ DE 1217391, Bredereck, H.; Effenberger, F. & Brendle, T., "Verfahren zur Herstellung von Tris-dimethylaminomethan (Process for the production of tris(dimethylamino)methane)", issued 1966-12-08, assigned to Bredereck, H.
- ^ Bredereck, H.; Effenberger, F.; Brendle, T.; Muffler, H. (1968). "Orthoamide. V. Synthese von Tris-dialkylamino-methanen" [Orthoamides. V. Synthesis of tris(dimethylamino)methane]. Chemische Berichte (in German). 101 (5): 1885–1888. doi:10.1002/cber.19681010541.
- ^ Kantlehner, W.; Maier, T.; Speh, P. (1979). "Tris(dialkylamino)methane und Tetraalkylformamidinium-Thiocyanate aus Bis(dialkylamino)acetonitrilen" [Tris(dialkylamino)methanes and tetraalkylformamidinium thiocyanates from bis(dialkylamino)acetonitriles]. Synthesis (in German). 1979 (5): 342–343. doi:10.1055/s-1979-28671. S2CID 97378246.
- ^ a b Kantlehner, W.; Stieglitz, R.; Hauber, M.; Haug, E.; Regele, C. (2000). "Orthoamide. LII. Beiträge zur Synthese von Orthocarbonsäureamiden" [Orthoamides. LII. Articles on the synthesis of carboxylic acid orthoamides]. Journal für praktische Chemie (in German). 342 (3): 256–268. doi:10.1002/(SICI)1521-3897(200003)342:3<256::AID-PRAC256>3.0.CO;2-G.
- ^ Bredereck, H.; Effenberger, F.; Simchen, G. (1961). "Reaktionsfähige Säureamid-Dimethylsulfat-Komplexe" [Reactive acid amide–dimethyl sulfate complexes]. Angewandte Chemie (in German). 73 (14): 493. Bibcode:1961AngCh..73..493B. doi:10.1002/ange.19610731407.
- ^ DE 2214497, Leimgruber, W. & Wick, A. E., "Verfahren zur Herstellung eines aminosubstituierten Methanderivates (Process for the manufacture of an amino-substituted methane derivative)", issued 1972-10-05, assigned to F. Hoffmann-La Roche & Co. AG
- ^ Weingarten, H.; White, W. A. (1966). "A novel amination reaction of carboxylic acid derivatives with tetrakis(dimethylamino)titanium". Journal of the American Chemical Society. 88 (4): 850. doi:10.1021/ja00956a049.
- ^ Kantlehner, W.; Haug, E.; Mergen, W. W.; Speh, P.; Maier, T.; Kapassakalidis, J. J.; Bräuner, H. J.; Hagen, H. (1983). "Ein Herstellungsverfahren für N,N,N′,N′,N″,N″-Hexaalkylguanidinium-chloride" [A manufacturing process for N,N,N′,N′,N″,N″-hexaalkylguanidinium chlorides]. Synthesis (in German). 1983 (11): 904–905. doi:10.1055/s-1983-30558. S2CID 93420838.
- ^ Kantlehner, W.; Speh, P.; Bräuner, H. J. (1983). "Eine einfache Synthese für Tris(dialkylamino)methane" [A simple synthesis for tris(dialkylamino)methanes]. Synthesis (in German). 1983 (11): 905–906. doi:10.1055/s-1983-30559. S2CID 101466032.
- ^ Bredereck, H.; Effenberger, F.; Bredereck, H. J. (1966). "Eine neue Synthese von Tetra(dimethylamino)äthylen" [A new synthesis of tetra(dimethylamino)ethylene] (PDF). Angewandte Chemie (in German). 78 (21): 984. Bibcode:1966AngCh..78..984B. doi:10.1002/ange.19660782113.
- ^ Wiberg, N.; Buchler, J. W. (1962). "Tetrakis(dimethylamino)äthylen: Ein starker Elektronendonator" [Tetrakis(dimethylamino)ethylene: A strong electron donor]. Angewandte Chemie (in German). 74 (14): 490–491. Bibcode:1962AngCh..74..490W. doi:10.1002/ange.19620741410.
- ^ Kantlehner, W.; Hauber, M.; Vettel, M. (1996). "Orthoamide. IL. Umsetzungen von Orthoamid-Derivaten mit Schwefel und Selen, Synthesen von 1,3-Thiazol- und 1,3-Selenazolderivaten" [Orthoamides. XLIX. Reactions of orthoamide derivatives with sulfur and selenium, syntheses of 1,3-thiazole and 1,3-selenazole derivatives]. Journal für praktische Chemie (in German). 338 (1): 403–413. doi:10.1002/prac.19963380180.