Tellurium tetraiodide
| |
| Names | |
|---|---|
| Other names
tellurium(IV) iodide
| |
| Identifiers | |
3D model (JSmol)
|
|
| ChemSpider | |
| ECHA InfoCard | 100.029.282 |
| EC Number |
|
PubChem CID
|
|
| UNII | |
CompTox Dashboard (EPA)
|
|
| |
| |
| Properties | |
| TeI4 | |
| Molar mass | 635.218 g/mol |
| Appearance | black crystals |
| Density | 5.05 g/cm3, solid |
| Melting point | 280 °C (536 °F; 553 K) |
| Structure | |
| orthorhombic | |
| Hazards | |
| GHS labelling:[1] | |
 
| |
| Danger | |
| H302, H312, H314, H332 | |
| P260, P261, P264, P270, P271, P280, P301+P312, P301+P330+P331, P302+P352, P303+P361+P353, P304+P312, P304+P340, P305+P351+P338, P310, P312, P322, P330, P363, P405, P501 | |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
| |
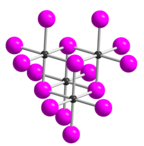
Tellurium tetraiodide (TeI4) is an inorganic chemical compound. It has a tetrameric structure which is different from the tetrameric solid forms of TeCl4 and TeBr4.[2] In TeI4 the Te atoms are octahedrally coordinated and edges of the octahedra are shared.[2]
Preparation
[edit]Tellurium tetraiodide can be prepared by reacting Te and iodomethane, CH3I.[2] In the vapour TeI4 dissociates:[3]
- TeI4 → TeI2 + I2
It can be also obtained by reacting telluric acid with hydrogen iodide.[4]
- Te(OH)6 + HI → TeI4 + I2 + 6 H2O
It can also be obtained by reacting the elements, which can also produce tellurium diiodide and tellurium monoiodide, depending on the reaction conditions:[5]
- Te + 2 I2 → TeI4
- TeI4 → TeI2 + I2
Properties
[edit]Tellurium tetraiodide is an iron-gray solid that decomposes slowly in cold water and quickly in warm water to form tellurium dioxide and hydrogen iodide.[6] It is stable even in moist air and decomposes when heated, releasing iodine. It is soluble in hydriodic acid to form H[TeI5] and it is slightly soluble in acetone.[4]
Tellurium tetraiodide is a conductor when molten, dissociating into the ions TeI3+ and I−. In solvents with donor properties such as acetonitrile, CH3CN ionic complexes are formed which make the solution conducting:[3]
- TeI4 + 2 CH3CN → (CH3CN)2TeI3+ + I−
Five modifications of tellurium tetraiodide are known, all of which are composed of tetrameric molecules.[7] The δ form is the most thermodynamically stable form. This is structurally derived (as well as the α, β and γ forms) from the ε form.
References
[edit]- ^ "Tellurium tetraiodide". pubchem.ncbi.nlm.nih.gov. Retrieved 13 December 2021.
- ^ a b c Greenwood, Norman N.; Earnshaw, Alan (1997). Chemistry of the Elements (2nd ed.). Butterworth-Heinemann. ISBN 978-0-08-037941-8.
- ^ a b Inorganic Chemistry,Egon Wiberg, Arnold Frederick Holleman Elsevier 2001 ISBN 0-12-352651-5
- ^ a b Handbuch der präparativen anorganischen Chemie. 1 (3., umgearb. Aufl ed.). Stuttgart: Enke. 1975. p. 435. ISBN 978-3-432-02328-1.
- ^ Hagen, A. P. (2009-09-17). Inorganic Reactions and Methods, The Formation of Bonds to Halogens (Part 1). John Wiley & Sons. ISBN 978-0-470-14538-8.
- ^ Tellurium(IV) iodide, 99% (metals basis) at AlfaAesar, accessed on 2013-12-17 (PDF) (JavaScript required).[dead link]
- ^ Riedel, Erwin; Janiak, Christoph (2011). Anorganische Chemie: Zusatzmaterial online. Studium (8. Aufl ed.). Berlin: de Gruyter. ISBN 978-3-11-022567-9.
