Talk:Thiocyanate
| This article is rated Start-class on Wikipedia's content assessment scale. It is of interest to the following WikiProjects: | |||||||||||
| |||||||||||
Thiocyanate and Happiness
[edit]On most articles I see about molecular compounds (polyatomic ions or neutral molecules), there's a fire diamond and toxicity warnings. I figured that thiocyanate would have toxicity warning posted because of the C≡N bond, but the attached sulfur could change the toxicity completely. Does anyone happen to know the safety concerns about this ion? Kyoobur9000 (talk) 18:54, 30 September 2012 (UTC)
- The reason that there isn't is that they are not that toxic. Also, look at the individual thiocyanates for definite toxicological data, as not all compounds are created equally. Thiocyanates, SCN-, do not generate cyanides, CN- ions.JSR (talk) 20:50, 27 September 2012 (UTC)
Lewis Structure
[edit]I have changed the diagram to one where the carbon is double-bonded to both sulfur and nitrogen. Here, the negative formal charge is on the nitrogen atom, which, as nitrogen is the least electronegative of the atoms, is correct. The previous structure had nitrogen triple-bonded, which places the negative formal charge on sulfur, which is incorrect. Bochum (talk) 04:55, 2 June 2008 (UTC)
- It's not that simple:
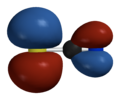
- if you look at the HOMO of [SCN]−, it has larger lobes on sulfur than on nitrogen
- the electric potential surface shows similar potential on sulfur and nitrogen (bit more on N but not massively so)
- the ionisation potential surface shows electrons are most easily removed from sulfur, so thiocyanate is often nucleophilic at sulfur
- thiocyanate reacts at either S or N (depending on conditions and what it's reacting with)
- All these facts mean that a pair of resonance structures would be better.
- p.s. if you're going to be adding more chemical structures, take a look at Wikipedia:WikiProject Chemistry/Structure drawing.
-
HOMO
-
electric potential surface
-
ionisation potential surface
I just did some calculations in Spartan. It gives the following charges:
Nitrogen
Electrostatic: −0.67
Mulliken: −0.58
Natural: −0.60
Sulfur
Electrostatic: −0.71
Mulliken: −0.56
Natural: −0.55
Carbon
Electrostatic: 0.37
Mulliken: 0.14
Natural: 0.15
So you can see that there's very little difference between the charge on nitrogen and the charge on sulfur, and therefore it does not make sense to give only one resonance structure in the article.
Ben (talk) 11:19, 2 June 2008 (UTC)
SCN or M−NCS.
[edit]SCN or M−NCS. 27.63.21.151 (talk) 07:58, 25 June 2022 (UTC)
Thiocyanate Complexes have Mostly Blood Red Color.
[edit]Thiocyanate color 37.111.128.188 (talk) 15:21, 14 November 2022 (UTC)