Talk:Periodic table/Archive 12
| This is an archive of past discussions about Periodic table. Do not edit the contents of this page. If you wish to start a new discussion or revive an old one, please do so on the current talk page. |
| Archive 5 | ← | Archive 10 | Archive 11 | Archive 12 | Archive 13 | Archive 14 | Archive 15 |
Recent attempts to change the periodic table (Scerri 2020)
Here's a link to the latest article by Eric Scerri, a world authority on the periodic table.
Here are some extracts from, and my commentary on, this interesting piece:
Philosophy v pragmatism?
The focus of the paper is philosophy rather than pragmatism. (p.2)
I suggest a need to focus on philosophy and pragmatism.
Focusing on just one results in needless arguments, including of the kind I used to make.
Insisting on one PT
Scerri writes:
- "There is no need to insist on the periodic table having a format that is suited mainly for the purposes of the chemical community and for chemical educators." (p. 6)
As far as I know, no one has made such an insistence. It is rather a case of people insisting on such a table within a particular sub-context or interest dependence, and usually not making this clear enough.
Who owns the PT?
Scerri writes:
- "The periodic table has now become as much the property of physicists, geologists, astronomers and others as it is of its chemical originators." (p. 7)
This is a contentious generalisation.
I suggest the periodic table, in the first instance, remains the organising icon of chemistry. Thus, here is what Scerri said in the 2nd (2020) edition of his Red Book:
- "…it helps to remember that, when all is said and done, the periodic table remains primarily in the domain of chemistry, although the relationship between chemistry and the underlying explanation from physics remains as the underlying theme… (p. ix)"
Rather than becoming a shared commodity, the periodic table concept has been borrowed, adapted, tailored and presented in various different guises—including the 15-element wide f-block version—by the physicists, geologists, astronomers and others.
As Scerri rightly says:
- "It becomes increasingly clear that there may not be any such thing as one optimal table in a purely objective sense. The question seems to depend on what criteria are considered and, most importantly perhaps, on whether one favours chemical or physical criteria or general didactic considerations." (p. 12)
To this commendable end, he goes on:
- "We should accept that a degree of convention must be used in selecting a periodic table that can be presented as perhaps the best possible table that combines objective factors as well as interest dependence." (p. 14)
Quite so, having regard to the priorities of each interest group.
The Madelung Rule
- "In any case, it is interesting to see that Pyykkö admits that it is surprising, in view of the relativistic effects, that the Madelung rule survives so well all the way up to atomic number 172." [!] (p. 8)
As far as the MR anomalies/symmetry breaking we observe in real life are concerned, the analogy is to an aeroplane experiencing turbulence. The flight path always returns to normal, after each turbulence episode.
The MR can also be regarded as the "spine" underlying the pattern of free atom electron configurations. The spine has bumps, dips, and knobbly bits on it, but still runs "true", so to speak.
Group 3
- "While the majority of textbook and other periodic tables in the 18-column format show the elements of this group as scandium, yttrium, lanthanum and actinium, a significant number of more recent tables feature the last two elements as lutetium and lawrencium instead." (p. 10)
Serving the largest audience
Scerri seems to go off-message in his conclusion, with references to:
- "…the format of the periodic table that serves the greatest number of periodic table users including students, instructors and practising chemists alike"; and
- "…how the periodic table is presented to the widest possible audience of users." (p. 15)
Surely the result of these notions will be a periodic table that attempts to cater to everyone but pleases no one?
Going off-message at this point is peculiar, since he earlier wrote:
- "It becomes increasingly clear that there may not be any such thing as one optimal table in a purely objective sense. The question seems to depend on what criteria are considered and, most importantly perhaps, on whether one favours chemical or physical criteria or general didactic considerations." (p. 12)
Accordingly, give me a tailor-made PT anytime, whether that is 14CeTh, 15LaAc, or 14LaAc, Adomah, AAE, Janet or some other variation, as long as the applicable context is set out.
The bugaboo of the split s-block
Scerri notes the periodic table is generally depicted with helium in group 18, and this splits the s-block (p. 11).
This is one of those things—the split s-block—that effectively all chemists (to a first approximation) do not lose any sleep over.
Same goes for the split d-block, which is less visible.
That said, better chemists keep both of these interesting aspects of the PT in mind.
It is like what Jones says:
- "Scientists should not lose sleep over the hard cases. As long as a classification system is beneficial to economy of description, to structuring knowledge and to our understanding, and hard cases constitute a small minority, then keep it. If the system becomes less than useful, then scrap it and replace it with a system based on different shared characteristics." Jones 2010, Pluto: Sentinel of the outer solar system, Oxford University Press, p. 171).
The anomalous first period
- "Another attractive feature of the left-step table is that it restores regularity and perhaps even balance to the otherwise awkwardly shaped traditional periodic table representation. More significantly than such aesthetic considerations, this table provides greater regularity in depicting every single-period length as repeating once as, 2,2,8,8,18,18,32,32. Meanwhile, the traditional table features an anomalous first period that, unlike all subsequent ones, does not repeat in length to give a sequence of 2,8,8,18,18,32,32. (p. 13)
I don't understand why the lack of repetition of the length of the first period is regarded as anomalous. There is no first principles derivation being breached here, as far as I know. It only means that, from a chemistry perspective, it is more meaningful to break the periods after the end of the noble gases. At the same time, the left step table is still good for its particular uses.
Thorium
- "Needless to say, the characterization of these blocks of the periodic table is only approximate, just as the assignment of electronic configurations to atoms represents an approximation. Moreover, one may readily concede that an element such as thorium does not actually possess any f-orbital electrons and yet it is classified as being among the f-block elements even in all four of the periodic table representations shown in figures 10 to 13.
--- Sandbh (talk) 00:44, 19 July 2020 (UTC)
I finally got around to reading this fascinating piece, and offer here my comments, which I am also emailing to Mr. Scerri.
- Page 2, section 2, paragraph 2, sentence 1: "an eight column" should be "an eighth column"
- Page 13, paragraph 1 & 2. In the phrases "clearly illogical situation" and "restores logic to the situation", it seems to me that this is not an issue of violating and restoring "logic" in the sense of deduction, but rather an issue of an "irregularity" violating a simplistic pattern which turns out to be perfectly "regular" according to a slightly more complex pattern.
- Page 13, paragraph 2, last sentence. In "completely redundant in Schwartz's view", the term "redundant" doesn't seem to be quite correct, at least to my ears. It isn't "redundant" in the sense of "repeating something else", but rather it is "superfluous" or "unnecessary". But perhaps this is merely a difference between my American dialect and a more British one where an employee becomes "redundant" when they are no longer needed, whether or not their work duplicates that of another employee.
- Page 18, paragraph 1, lines 1-2: " evolution spoils any hope ... bound to change as time evolves". I don't think anyone would contend that time itself "evolves". Rather, time passes by as other things may or may not change.
- General comment: Just as the 50-column table provides insight into the construction of a 32-column table, and the 50- and 32-column tables provide insight into the construction of an 18-column table, it might be interesting to apply insights from 50-, 32-, and 18- column table to consider what might be the "best" 8-column table, and then consider what its deficiencies might be that make an 8-column format impractical. This is treated in one of the footnotes; that might be all it deserves, but I would find a more extended discussion interesting.
— YBG (talk) 03:42, 1 August 2020 (UTC)
IUPAC: an endorsement on group 3 (1988)
Opening statement by Double sharp
I apologise for raising this again. I beg forgiveness for it because of the title. So, this may be taken as a start of a new and totally policy-based and drama-free discussion.
In rushing to start an RFC above based primarily on the sources debating the scientific arguments (which I now realise isn't really that relevant for Wikipedia), I appear to have overlooked what the most important organisation here has actually said about this in the past. In fact, that appears to have been completely overlooked in all the arguing back and forth over the past few months (mostly between myself and Sandbh). So I remedy this now.
In the comments under a 2019 article by Eric Scerri (for those who do not know him, he is a world authority on the history and philosophy of the periodic table, as our article on him notes), Scerri himself adds that IUPAC already endorsed the Lu under Y form in a 1988 report.
| “ | Thirdly, I should also mention that figure 3 [Sc-Y-Lu, 32 column] that I call an optimal table, was already endorsed in an earlier IUPAC report, E. Fluck, New Notations in the Periodic Table, Pure and Applied Chemistry, 60, 3, 431-436, 1988. | ” |
The periodic table the paper gives in Fig. 3 is only partially drawn, but it clearly shows the La-Yb / Ac-No f block characteristic of the Lu under Y form. And on the last page we find the following text:
| “ | The Elements of the Scandium Group
In the "Red Book" which will appear in 1988 the same arrangement was chosen for the elements of the scandium group as in the periodic table as originally proposed by CNIC and subsequently published by VCH Verlagsgesellschaft, Weinheim. It is a compromise. According to the electron configurations of the elements, the scandium group consists of the elements This was pointed out as early as 1959 by L.D. Landau (ref. 20) and later by other authors (ref. 13, 14, 20 to 25). Most periodic tables in textbooks and classrooms, however, list Sc, Y, La, and Ac as elements of the scandium group and designate the elements Ce to Lu and Th to Lr as lanthanides and actinides, respectively. The historical background for this arrangement is given in a paper by W.B. Jensen (ref. 21). Based upon their electronic configurations and their chemical and physical properties, the elements La to Yb and Ac to No should be inserted between barium and lutetium and between radium and lawrencium or for practical reasons be listed at the bottom of the table. The series La to Yb and Ac to No then, however, cannot be named correctly as lanthanides and actinides since they contain the elements lanthanum and actinium and not only elements similar to lanthanum and actinium as is purported by the ending -ide (or -oid according to an earlier IUPAC recommendation). |
” |
There apparently was no Red Book published in 1988: maybe what is meant is what became the 1990 Red Book, but I cannot get the relevant parts of its periodic tables clearly on Google Books' snippet view. There seem to be multiple ones on pp. ii, 282, and 283. I have asked at WP:REX.
However, granting that does also raise a few questions. If it was decided, then why is IUPAC not using Sc-Y-Lu now even internally, calling all the lanthanides and actinides group 3 elements in the JWP reports? Why does the 2005 Red Book show a Sc-Y-* table? Was the decision rescinded? Why is there a new task force since 2015? (Although I suppose this one is easily answerable: it could be to relook at a previously decided situation even if the earlier decision was not rescinded.)
A reasonable interpretation is that it is something like the spelling of aluminium, in which IUPAC made a decision (it was the only official spelling in 1990–3, so around the same time), and then had to allow "aluminum" as an alternative (not the first one though) when people didn't want to follow. And for Wikipedia that situation is already interesting, as we do in fact follow IUPAC's "aluminium" consistently there to avoid lame edit wars, despite the fact that the 2005 Red Book actually does not endorse "aluminium" anymore (it writes that as the name of the element, but adds a footnote that reads "The alternative spelling ‘aluminum’ is commonly used.") I do not have proof of that, but can find no other explanation that reconciles a past IUPAC endorsement with IUPAC currently not using the Lu under Y form and having a task force ongoing to decide the matter.
Therefore: currently we seem to be in a limbo where IUPAC is relooking at the situation: so, should we follow
- Lu under Y, which has been endorsed by IUPAC, but in an endorsement that doesn't seem to translate to actual use anymore (which parallels "aluminium" where we follow the old IUPAC endorsement from almost the same period anyway)?
- The placeholder * under Y, which IUPAC currently shows, but takes care to say "IUPAC has no recommendation for a specific form of the periodic table", and that we know the new task force is not considering?
- or La under Y, which appears as of the 2010s in a plurality of general chemistry textbooks, but no longer a majority as it used to have (according to the IUPAC survey, statistics listed above)?
How do others see this?
I would, as you all know by now, favour option 1. For here, not because of the science, but because of the precedent of WP:ALUM. In that case, the situation was otherwise rather impossible to resolve, as British sources obviously use "aluminium" and American ones obviously use "aluminum". So we followed an old IUPAC decision, even despite its lack of actual adherence later by IUPAC itself, and in spite of the fact that you'll always find more "aluminum" and "cesium" than "aluminium" and "caesium".
So, that seems to me rather the same situation: the situation is rather impossible to resolve, as comparing textbooks shows a distribution that changes significantly depending on decade, even if La always manages a plurality; comparing periodic tables on Google Images rather favours *; and counting articles arguing about the matter rather favours Lu. There is also an old IUPAC decision, although IUPAC itself does not seem to adhere to it anymore.
And of course, it should be kept in mind that anything we decide now will be provisional. IUPAC has an ongoing task force since 2015 and it should come to another decision, which surely we will follow on Wikipedia whatever it turns out to be.
So: it seems to make the most sense to me to follow what IUPAC actually endorsed in 1988, rather than what they show now but take care not to say they endorse, when what we are waiting for is none other than another IUPAC endorsement.
And it sidesteps any arguments about which sources are the most relevant. We can argue all day about whether the most relevant sources for periodic tables are general chemistry textbooks or the posters you usually see and get off the Internet, about whether the relevant sources are the articles focusing on the matter or what chemists standardly use, etc. etc. etc. But here we have an actual past endorsement from IUPAC, which is surely the most relevant organisation for this.
Now, there is another writing-related reason why I would prefer Lu under Y. It is a sort of WP:IAR. I will now try to express it briefly in a drama-free way. I will rewrite if anyone objects.
The problem is that when it comes to explaining periodicity, which this article should do, showing any form that is not Lu creates a contradiction. The problem is inherent in what reliable sources say when it comes to the science. I will restrict myself to three observations.
- Reliable sources agree that actually the gas-phase ground-state configurations aren't very relevant for chemistry. Most chemical processes do not involve gas-phase atoms. Actually, in chemical environments configurations change significantly. The most famous example is probably that in transition metal complexes, even neutral ones, the configuration likely has no outer s electrons, even though that is not what any transition metal atom but palladium is in the ground state. This understanding is, for example, in Greenwood and Earnshaw 2nd edition, page 960 (table of group 4 complexes; neutral ones are labelled d4). And for example Glenn T. Seaborg noted this difference: "It is important to realize that the electronic structures listed in Table 6 are those of the neutral (unionized) gaseous atoms, whereas it is the electronic structure of the ions and compounds that we are chiefly concerned with in chemistry".
- Moreover, reliable sources usually do not even define blocks, much less differentiating electrons. Neither is actually in the index for Greenwood and Earnshaw (they do mention blocks, but they don't define what they are). Cotton and Wilkinson only refer to differentiating electrons twice, and in neither case do they actually give a definition that sorts out the problematic cases like vanadium d3s2 versus chromium d5s1. Not to mention that reliable sources agree that electrons are indistinguishable: Richard Feynman says it, and it's completely standard chemistry that the electrons in a covalent bond are indistinguishable (an atom can't tell which one belonged to it and which one didn't). That does not sit well with the idea of differentiating electrons.
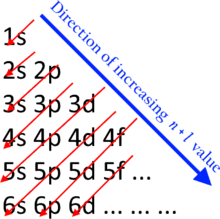
- And reliable sources generally derive periodicity from the Madelung rule. That demands in the sixth row "6s, 4f, 5d, 6p", as in the graphic to the right. Even the IUPAC document mentioned above says that: "CNIC rejected the proposal [for different group numbers] arguing that 1) the code of elements is rather an illustration of the Aufbau principle from which a periodic system is to be developed" (my italics, which are hopefully considered OK to use, as Sandbh has previously objected to bolding).
Now, let's not argue about whether these are right. The important thing is: they are supported by sources, they should go in. The problem is that since the La table is, as the article notes, based on gas-phase ground-state configurations and differentiating electrons, it looks rather strange to explain periodicity following sources that note that they are not relevant, and then focus on a table that came from there (and only partly, as while Ac anomaly is reflected Th is not). And it is very strange to mention the Madelung rule as the basis for the PT, and then show a table that does not follow it, and then apologise for it by referring to sources for 4f becoming non-hydrogenic at La already.
Therefore, I claim: for the twin reasons of
- the past IUPAC endorsement from 1988 of Lu under Y;
- the writing problem that appears if you don't pick Lu under Y;
Lu under Y is better for WP.
But, of course I will follow whatever consensus results. Double sharp (talk) 13:46, 1 August 2020 (UTC)
- Sounds well reasoned and drama-free to me. A few comments:
- It is not surprising that a body like the IUPAC would make an endorsement and then forget about it. They've got no language police or WP:wikilawyers.
- The parallel with WP:ALUM is instructive and convincing.
- There is bound to be a fair amount of pushback so we should have our consensus firmly in hand before thinking about changes
- It would be wise to delay if there is a significant chance the Group 3 group will deliver its decision in less than a year, especially if there's a chance they'll come up with something different.
- And of course, I'll abide by whatever consensus is reached. YBG (talk) 22:18, 1 August 2020 (UTC)1
- @YBG: Alas, I don't know how the group 3 project is going. They do post progress updates, but quite sporadically. The last one is from December 2019 and the previous one is from March 2018. As both La and Lu supporters are on it, it could honestly go either way.
- As for getting a consensus: that's why I'm raising it here. ^_^ Double sharp (talk) 03:25, 2 August 2020 (UTC)
Observations by Sandbh
I'll address Double sharp's observations in sequential order.
IUPAC: an endorsement on group 3 (1988)
Untrue. No such endorsement was ever made.
Scerri himself adds that IUPAC already endorsed the Lu under Y form in a 1988 report.
Untrue. If it were, there would be no reason for IUPAC to agree to the establishment of the Group 3 project, chaired by Scerri.
The report Scerri refers to is here. A careful reading of the report shows that it is an historical review of the processes that led to the current group numbering system. That is all. It does not have the status of an IUPAC recommendation. Rather, it was published in IUPAC's journal, Pure and Applied Chemistry. That does not mean anything; you can find varying formats of periodic tables in this journal, for example, including the La form.
Towards the end of the article Fluck makes some passing comments about group 3. He notes that the *-** form is a compromise. This is a personal opinion; he does not give a citation. Then again, Philip Ball, former editor of Nature, who is on the IUPAC Group 3 project, opined that, "Chemistry is about compromise."
Fluck correctly says, "Most periodic tables in textbooks and classrooms…list Sc, Y, La, and Ac as elements of the scandium group and designate the elements Ce to Lu and Th to Lr as lanthanides and actinides, respectively.
In support of his personal opinion Fluck notably cites Landua & Lifshitz, and Jensen. L&L are not reliable as I noted here.. Jensen was described by Scerri as too selective in his arguments.
Fluck's article was never "endorsed" by IUPAC. It was an historical review, nothing more.
Textbooks, Google Images, counting articles
Double sharp writes: "…comparing textbooks shows a distribution that changes significantly depending on decade, even if La always manages a plurality; comparing periodic tables on Google Images rather favours *; and counting articles arguing about the matter rather favours Lu.
The 2010's text-book statistics are La 16 (~50%); Lu 6 (~18%); *-** 11 (33%). Google image searches are plagued by bias arising from the false impression that the IUPAC *-** table is “official”, and unreliable sources.
There are some arguments in the literature suggesting the Lu form looks better in some cases. Many of these are one-shots based on a single property. As I noted, Jensen had a red-hot go at corralling these arguments in 1982, in support of Lu in group 3 but his effort failed to gain traction. He was criticized by Scerri, (chair of the IUPAC Group 3 project group) and Parsons (2018, p. 143), for being too selective in his arguments. There was Holden (1985) who looked at seven properties but estimated the argument of Landau & Ligshitz (1958) to be the strongest. As noted, Landau and Lifshitz, are unreliable.
The situation is rather impossible to resolve.
There is nothing needing to be resolved. The La form continues to be the most popular form in the literature. As Scerri (2020) notes: "…the majority of textbook and other periodic tables in the 18-column format show the elements of this group as scandium, yttrium, lanthanum and actinium."
1. Reliable sources agree that actually the gas-phase ground-state configurations aren't very relevant for chemistry.
Reliable sources agree gas-phase ground-state configurations are a highly successful approximation.
2. Reliable sources usually do not even define blocks
Reliable sources usually organise the elements on the basis of blocks.
3. Reliable sources generally derive periodicity from the Madelung rule.
- Correct, noting the MR does not demand anything.
- Reliable sources usually note there are 19-20 deviations from the MR.
- Reliable sources usually note the appearance of an f electron does not occur until Ce.
--- Sandbh (talk) 04:54, 2 August 2020 (UTC)
PS: @Double sharp: IUPAC Red Book 1990 here. Check out the 32-column Lu form, at page 283! Sandbh (talk) 05:12, 2 August 2020 (UTC)
@Sandbh and YBG: I will now address Sandbh's observations dispassionately.
Endorsement. As I see it, Sandbh's interpretation seems somewhat at odds with what reliable sources say.
I quoted the IUPAC 1988 report above: it explicitly says "the scandium group consists of the elements Sc, Y, Lu, Lr", and goes on to justify it by referring to earlier work by Landau and Lifschitz and by Jensen, and by referring to electron configurations as well as chemical and physical properties. And I quoted how Eric Scerri, who is on the current IUPAC project, sees this statement: as an endorsement of the form with Lu under Y. Scerri, being on the current IUPAC project, surely will be well-placed to make a statement about what this document is.
Moreover, the report is only stated to be prepared for publication by E. Fluck. As can be seen from the title page, it rather comes from the IUPAC Inorganic Chemistry Division. And it goes on to state that "this form of the periodic table will therefore be set out in the Appendix to the new edition of the IUPAC Red Book (Nomenclature of Inorganic Chemistry)". That seems like more than Fluck's personal opinion.
And indeed, Sandbh has kindly linked to the 1990 Red Book. On p. ii, it shows a Sc-Y-* form. However, as Sandbh noted above: on pp. 281–3 (the very appendix in question) it shows an 8-column, then an 18-column, then a 32-column periodic table. The 8-column one is Sc-Y-*, as in the cell reading "La*" a footnote is given reading "including lanthanoids (57-71)", and the same for the actinoids. The 18-column one is explicitly Sc-Y-* (with La-Lu* below Y). But: the 32-column one is a Sc-Y-Lu table, exactly in accordance with what the 1988 IUPAC report says. Therefore, one can see that not only was the 1988 report an endorsement of the Lu form, but it translated at least partially to actual usage.
While Fluck does use the word "compromise", he does not refer to the Sc-Y-* form. In fact he goes on to talk about how the f elements fit into the table if the scandium group is considered to be Sc, Y, Lu, Lr. And indeed mentions that many tables show Sc, Y, La, and Ac in the scandium group; however, his language is clearly not endorsing this. And even if Sc-Y-* was in fact the "compromise" mentioned (which is not at all clear from Fluck's wording), it remains that in the 32-column form where no compromise is possible without "stretchy scandium and yttrium", IUPAC shows group 3 as Sc, Y, Lu, and Lr.
There are clearly any number of reasons why IUPAC should now want to relook at its decision. Perhaps it is because new scientific evidence has appeared since 1988, say, and they want to examine that. Or perhaps, it is because the issue got fresh publicity. Or perhaps, it is as YBG said and they simply forgot about their earlier decision. I obviously do not know what the real reason is, but the fact that it is easy to come up with plausible ones rather seems to be at odds with Sandbh's statement that "If it were [true that IUPAC had endorsed the Lu form in this 1988 report], there would be no reason for IUPAC to agree to the establishment of the Group 3 project, chaired by Scerri."
Sources in the literature. Regarding Sandbh's statement that "the La form continues to be the most popular form in the literature", the IUPAC survey shows that 16 out of 33 undergraduate chemistry textbooks from the 2010s showed the La under Y form. That is indeed a plurality of 48%, but it surely cannot be taken as decisive.
Sandbh also argues that the Google Images results are plagued by bias and speculates on why they show what they do. But one can easily raise the very same concerns against textbooks. When a textbook shows the * form, is it because its authors analysed the situation and decided that was the best, or is it because they interpreted IUPAC currently showing this form as an endorsement, when they quite clearly state that it isn't? When a textbook shows the Lu form, is it because its authors really analysed the situation themselves, did they decide they were convinced by Jensen, or did they just do it because it looks neater? When a textbook shows the La form, is it because it authors really analysed the situation, or is it just because they were not aware that a dispute existed in the first place? The last option is in fact quite probable: remember, in Jensen's own 1982 article that the IUPAC report cites (doi:10.1021/ed059p634), he writes:
| “ | Indeed, in talking with his fellow chemists, the author discovered that none of them was aware of the evidence favoring the reassignment of lutetium and lawrencium or indeed that there ever was any question about their placements (a category in which the author must include himself until very recently). | ” |
The bottom line is: we are not here to second-guess why a source made a decision. We can only talk about that if they tell us, and they generally don't. What matters for Wikipedia is what they show. And that differs quite strongly depending on what sources you focus on. That is why I consider the situation undecidable on sources alone: textbooks have a plurality for La (but not a majority), Google Images searches give a majority for *, and articles arguing the issue give a majority for Lu. That's exactly the kind of situation where, as with WP:ALUM, we are willing to use old IUPAC decisions even if they are not actually adhered to completely by IUPAC itself.
Analysis of sources. The idea that periodic tables on the internet are "unreliable" also overlooks the problem that the periodic table is not solely the province of undergraduate chemistry textbooks. It's trivially easy to find tons of them in books and online, and especially the online ones are what students will most likely be looking at.
Finally, Sandbh's characterisation of the Lu arguments as "one-shots" is not something that, as far as I am aware, can be found in the literature. It should also be noted that many of these one-shot arguments in fact appear in Jensen's article. Merz and Ulmer, for instance, was called "another pop gun" by Sandbh in Wikipedia talk:WikiProject Elements/Archive 44. Yet it is one of the arguments that is explicitly mentioned in Jensen's paper: it is mentioned as "d-Block-like structure for conduction band" and cited as reference (13). So not only is Sandbh's characterisation rather at odds with how the argument was treated and reliable sources, it seems to suggest that an argument is not worthy of consideration (Merz and Ulmer) as long as it appears alone, but when it is combined with many other ones (as Jensen did) it becomes fair game. That seems strange.
If Sandbh personally is unconvinced by the Lu arguments, that is his right. Nevertheless, they are referred to in reliable sources, and mentioned as the basis for the IUPAC endorsement. We should be following the reliable sources rather than trying to discredit them by our own analyses. Any discrediting can only be done here if it comes from other reliable sources.
An example of this is where Sandbh refers to his own analysis at Wikipedia talk:WikiProject Elements/Archive 48#Landau & Ligshitz (1958): Redux to justify his contention that Landau and Lifshitz's Course of Theoretical Physics is not a reliable source regarding the matter. That is brought in despite the fact that it is not the important thing here (it's just another source); it is rather just a source that the IUPAC 1988 report cited. And even if it was the important thing, it remains that this is a world-renowned textbook that got its authors the Lenin Prize of 1962. And while I am in personal agreement with him that what Landau and Lifshitz says does not go so far as to say that only Lu belongs in group 3 (because they just say Lu doesn't belong with the rare earths, and place La and Lu in a "platinum group" that is supposed to contain the filling of the 5d shell), and rather seems to be closer to saying La and Lu both belong there, I feel his conclusion is marred by the fact that it's WP:OR, against how the IUPAC report that is under consideration interpreted it, and that he refers to a "Landau and Lifshitz periodic table" that is not what Landau and Lifshitz actually gives.
| What Landau and Lifshitz give | What Sandbh in his own analysis calls a "Landau and Lifshitz periodic table" |
|---|---|
   |
 |
Again, I refer to the precedent of WP:ALUM, and the fact that 48%, however you slice it, is not a majority. It would even not be the first time that we followed a IUPAC endorsement even when the majority of authors do not follow it. Not only is there the precedent of WP:ALUM, there is also the precedent of the systematic element names. IUPAC recommended these ("ununennium" etc.), but they are not used much among the nuclear physicists and chemists involved in synthesising these elements. Despite this, we continue to follow IUPAC.
What reliable sources say. It is rather odd to say that reliable sources agree that gas-phase ground-state electron configurations are a highly successful approximation when I have shown sources that explicitly say that they have to be used with caution and that they are irrelevant. I quote them again below in a collapse box, without the bolding that Sandbh objected to before when I posted them. In fact I add one more very recent one.
Extended content
| ||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| ||||||||||||||||||||||||
We also have a big-name inorganic chemistry textbook acknowledging it. Greenwood and Earnshaw, it is true, puts gas-phase ground-state electron configurations in its tables. But they well understand that in chemical environments these configurations change. On page 960 they list a bunch of coordination complexes of the elements of group 4. There they refer to [Ti(bipy)3] and call it a d4 complex. Here, titanium is in the 0 oxidation state: it has not gained or lost any electrons. Yet they agree that here it does not show the d2s2 configuration that it has as a gas-phase atom in the ground state.
Since lanthanum is at issue, I also point to a recent article (doi:10.1039/C3CP50717C) showing that La may use its 4f orbitals for bonding despite them being unoccupied in a gas-phase La atom in its ground state. That is significant as it is one of the main arguments authors have used in support of Lu under Y: that La has low-lying non-hydrogenic f orbitals, whereas Lu does not. It appears in Jensen's 1982 paper.
As I mentioned, this is significant for the La form, as it stems from the ground-state gas-phase anomaly in La and in Ac. We must follow reliable sources, and that implies that we should say all of this about ground-state gas-phase anomalies being irrelevant for transition metals and that they can take different configurations when chemically bound. But that sits very badly with saying that the La form is based on ground-state gas-phase anomalies. It does not help the reader's understanding to say something is irrelevant and then justify a form shown based on what we have just said is irrelevant.
It is also, to me, very surprising to see Sandbh say that "reliable sources usually organise the elements on the basis of blocks", when reliable sources often do not even bother to define what a block is. On the rare occasion that a definition is actually offered, the reliable source in question generally does not follow it. Take this text for example, by A. Ramakrishna. It says "When the differentiating electron enters the d-orbital the elements are called d-block elements". Yet it lists zinc as a d-block element just the previous page, despite the fact that its differentiating electron does not enter the d-orbital (copper, the previous element, is [Ar]3d104s1; and zinc adds an s-electron to become [Ar]3d104s2). I do not see such an inconsistent definition as support for organising the elements on the basis of blocks.
Finally, reliable sources already support the idea that it is not at all important that the first f electron appears only in a gas-phase ground-state atom of cerium, because they agree that gas-phase ground-state electron configuration anomalies in the d and f elements are not relevant for chemistry, and that lanthanum has 4f involvement in the bonding already. I have quoted them above. Again: if Sandbh is unconvinced by them, that is his right, but we are supposed to be following the statements of reliable sources here. They say lanthanum is using its 4f orbitals for chemistry: we follow. In order to override what a source says for Wikipedia, you need to at least have a more recent source that refutes what it is saying.
I do not dispute that the gas-phase ground-state configuration is a useful approximation for the s and p elements, where they typically indeed appear in chemistry courses around the world. What reliable sources question their relevance for is when it comes to the d and f elements: lanthanum is one of them.
Writing problems. I once again note to finish off: periodicity is agreed to be based on the Madelung rule. IUPAC itself said so in the 1988 report being referred to. Now, the Madelung rule states that 4f comes before 5d. Is this really well explained by a table like this?

We would first be saying that 4f comes before 5d, as that is what the Madelung rule says. But then we would have to engage in some verbiage explaining why the table shows one 5d electron coming before 4f. And then we would have to find some way to explain why it doesn't also show two 6d electrons coming before 5d, given the electron configurations in the early part of the f block:
| La | Ce | Pr | Nd | Pm | Sm | Eu | Gd | Tb | Dy | Ho | Er | Tm | Yb |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 4f05d16s2 | 4f15d16s2 | 4f35d06s2 | 4f45d06s2 | 4f55d06s2 | 4f65d06s2 | 4f75d06s2 | 4f75d16s2 | 4f95d06s2 | 4f105d06s2 | 4f115d06s2 | 4f125d06s2 | 4f135d06s2 | 4f145d06s2 |
| Ac | Th | Pa | U | Np | Pu | Am | Cm | Bk | Cf | Es | Fm | Md | No |
| 5f06d17s2 | 5f06d27s2 | 5f26d17s2 | 5f36d17s2 | 5f46d17s2 | 5f66d07s2 | 5f76d07s2 | 5f76d17s2 | 5f96d07s2 | 5f106d07s2 | 5f116d07s2 | 5f126d07s2 | 5f136d07s2 | 5f146d07s2 |
While it's true that lanthanum adds a d electron rather than an f one, and so does actinium: so does thorium. The La under Y table, although stated to be based on ground-state gas-phase electron configuration anomalies, only really reflects two: La and Ac. Many other elements are simply not reflected, such as Th, Pa, U, and Np. The d block in fact has more such anomalies.
And it would then have to follow reliable sources and explain that actually it doesn't matter because ground-state gas-phase electron configuration anomalies for d and f elements don't mean anything much for real chemistry. And then follow reliable sources and say that the activity of 4f orbitals begins at lanthanum, therefore cancelling out all the explanations previously stated. At every sentence a "but this, but this, but this". Surely it will confuse the lay reader to say all of this and then walk it all back.
Whereas: if we made the change, we could simply use a table like this.
No contradiction. We say the Madelung rule says 4f comes before 5d, and we show a periodic table that shows that. No problem, no self-contradictions, and no need to drill down into all sorts of complicated things that we eventually have to follow reliable sources by declaring irrelevant anyway. Of course, we may discuss in the notes the "shifted" f block characteristic of the Sc-Y-La table, but it can be left in the notes and not to bamboozle a beginner just learning this from our article.
For this reason, I claim that sticking to the Sc-Y-La form would not help to improve Wikipedia. Although reliable sources have not switched en masse to Lu, they generally agree with the observations that those who support Lu in the literature make, and disagree with the observations that those who support La in the literature make. I might guess it's because the reliable sources were unaware that the dispute existed, but again, that's not the point. The point is that writing about chemical periodicity becomes very problematic with the Sc-Y-La form as a default because when justifying it you come to a head-on collision with what reliable sources agree on. Therefore, I plead WP:IAR:
| “ | If a rule prevents you from improving or maintaining Wikipedia, ignore it. | ” |
| — WP:IAR | ||
Combined with the fact that Lu under Y has had actual IUPAC endorsement according to Scerri (who, being on the actual IUPAC project, surely must know quite a bit about what IUPAC has or has not done), which makes this not even in the first place ignoring any rules about reliable sources, I consider the case for changing back to Lu under Y quite strong.
Conclusion. After reading Sandbh's comment I see no reason to change my stand on this, and continue to support the reversion to the 2016 situation of Lu under Y as a default form. That is based firstly on what seems to be an actual IUPAC endorsement, even if the extent of its usage is a bit small, because of the WP:ALUM precedent; and secondly because of the writing problems that result if you want to follow reliable sources but not give Lu under Y. Naturally, other forms will continue to be discussed where appropriate, and the decision should be relooked at if IUPAC's new project comes to a different conclusion.
Double sharp (talk) 07:09, 2 August 2020 (UTC)
Discussion by Michael D. Turnbull, YBG, Double sharp
Scerri has recently (March 2020) published online the preprint of an article [1] that appears to show the way his thinking is going. He advocates for a 32-column table essentially identical to the one immediately above (his Figure 10) and for the same reason: not wanting to split the d block. He suggests that an 18-column table should be less preferred but, if used for example for teaching purposes it should that of his Figure 4, which is a Sc-Y-Lu-Lr version with a pair of 14-element f sets shown below. My personal conclusion from all this is that we should wait as the matter may well be settled by the IUPAC working group within a few more months. Michael D. Turnbull (talk) 18:05, 2 August 2020 (UTC)
- +1 Sandbh (talk) 00:24, 4 August 2020 (UTC)
- Wondering if we are using the phrase "reliable source" here in the sense used here at WP. WP:RS Primarily deals with which sources can be cited on WP. Secondarily it helps us identify things that should be excluded because they don't appear in RS. And thirdly, relative prominence in RS should determine relative prominence in WP, lest we give it WP:UNDUE WP:WEIGHT.
- This last wikilink is most instructive. See especially the last two paragraphs of the section beginning here:
Keep in mind that, in determining proper weight, we consider a viewpoint's prevalence in reliable sources, not its prevalence among Wikipedia editors or the general public.
If you can prove a theory that few or none currently believe, Wikipedia is not the place to present such a proof. Once it has been presented and discussed in reliable sources, it may be appropriately included. See "No original research" and "Verifiability".- ~ YBG (talk) 01:40, 3 August 2020 (UTC).
- @YBG: Lu under Y is not something that "few or none currently believe". It is something that has been believed since 1892 by various chemists and has gotten the endorsement of the relevant organisation itself, IUPAC, in 1988. True, it is not the most common form in chemistry textbooks (21% of textbooks published since the turn of the millennium show it according to the IUPAC survey). But if you look at the statistics above, you will see that since no form has a majority anymore: anything as default produces an undue weight problem: it is unavoidable. That's why I favour saying "look, IUPAC said this in 1988".
- Especially when you take into account the following situation: although reliable sources don't have a majority showing the Lu table, they absolutely do support en masse the principles behind it. It is completely standard in the literature that the bases for the La table, which are gas-phase ground-state differentiating electrons, are irrelevant for the d and f block elements. I quoted four articles and one textbook to support that. And yet, it is completely standard in the literature that the basis for the Lu table, Jensen's concept of valence electrons and vacancies as well as periodic trends, is correct and important. The first is just HOMO/LUMO, and the second is well known to all freshmen. The Madelung rule is completely standard too, but it is only consistent with a Lu table because it says 4f comes before 5d. There are yet more, but I will just stop here. Giving the La table on Wikipedia means that the requirement of following reliable sources will force us to subvert it all the time simply because those reliable sources agree that its bases are incorrect. We are faced with a choice of the following alternatives:
- Stay with La under Y, with a 48% plurality of chemistry textbooks, but only a 1/6 minority of periodic tables from Google Images results, and against IUPAC's 1988 endorsement and against the majority of the sources focusing on the issue. And which will come in direct conflict with even the textbooks showing La under Y itself, because the bases of the La form are themselves often refuted by those textbooks.
- Go back to Lu under Y. As we had in 2016, as IUPAC endorsed in 1988, as they put in 32 column form in the 1990 Red Book. In complete agreement with the literature focusing on the issue. And in complete agreement with the points even textbooks using La make regarding the change of electron configurations of transition elements in chemical environments, which certainly ameliorates the problem of not following their table layout. In terms of Google Images results it is not worse, having about the same prevalence as the La under Y form.
- It seems to me that (2) is clearly better.
- As above: I looked at reliable sources, I quoted what they say directly. It is explicit enough without any need for my own analysis. Sandbh seems to be using his own WP:OR and selective analyses to try to discredit them. One can see that from how:
- Sandbh doesn't accept a statement from IUPAC in 1988 that goes "the scandium group consists of the elements Sc, Y, Lu, Lr", and a written statement from Scerri, the chair of the current IUPAC project on group 3, that says that it was an endorsement. Instead Sandbh says that "no such endorsement was ever made" in direct contradiction with the chair of the current IUPAC project on group 3 (Scerri), who is obviously a reliable source on what IUPAC did or did not endorse in the past.
- And how Sandbh attempts to discredit the Google Images result (with a 2/3 majority for * under Y, and about equal 1/6 shares for La under Y and Lu under Y each) of being "plagued by bias arising from the false impression that the IUPAC *-** table is “official”", but fails to note that a statement in Jensen's 1982 article quoted by me above immediately implies that a survey of textbooks that extends before his article of 1982 and the IUPAC endorsement of 1988 will be plagued by bias arising from chemists not having been very aware of this dispute (even though it was already known among the physicists then). That is indeed why I only refer to the figures from the 2000s and 2010s, as by then Jensen and WebElements would have roused chemists' attention to the fact that the issue exists.
- And how Sandbh dismisses Lu arguments in the journal literature as one-shots, never mind that that characterisation AFAIK appears nowhere in the literature, that Jensen included in his 1982 article exactly some of those one-shots, and that IUPAC referred to Jensen's article among other sources (including Landau and Lifshitz, see point 6 below) to justify their 1988 endorsement.
- And how Sandbh mentions that Scerri and Parsons considered Jensen's arguments selective, while not mentioning that Scerri and Parsons actually support Jensen's conclusions anyway, albeit for different reasons.
- As you know, Scerri has acknowledge that the argument presented by him Parsons is unsuccessful. Sandbh (talk) 00:24, 4 August 2020 (UTC)
- And how Sandbh claims that reliable sources agree that gas-phase ground-state configurations are a highly successful approximation, when I have managed to produce four articles and one textbook substantiating my point that they are not so for the transition elements.
- And how Sandbh attempts to discredit the reliability of a renowned textbook on physics (Landau and Lifshitz) on this matter, on the grounds of his own WP:OR analysis that contradicts how the IUPAC report interprets Landau and Lifshitz's statement, and that involves drawing a "Landau and Lifshitz periodic table" that appears nowhere in Landau and Lifshitz.
- Is this neutral? Is this correctly reflecting the sources?
- We are waiting for IUPAC, indeed. We have no indication of when they will say something, given the sporadic updates of the project, and given that Scerri has been using this argument to advocate Sc-Y-Lu since at least 2009. And indeed, though Scerri is the chair of the current IUPAC task group on group 3, he was complaining about things in the very 2019 article that alerted me to the fact that IUPAC did endorse Sc-Y-Lu in 1988 already:
| “ | Why will IUPAC not see things quite so simply? That’s a big and complicated question which I can only touch upon here. Like many organizations with rules and regulations, when push comes to shove, decisions are made by committees. As a result, the science takes second place while the various committee members vie with each other and ultimately take votes on what periodic table they should publish. Unfortunately, science is not like elections for presidents or prime ministers, where voting is the appropriate channel for picking a winner. In science there is still something called the truth of the matter, which can be arrived at by weighing up all the evidence. The unfortunate situation is that IUPAC cannot yet be relied upon to inform us of the truth of the matter concerning the periodic table. In this respect there is indeed an analogy with the political realm and whether we can rely on what politicians tell us. | ” |
| — Eric Scerri | ||
- That's why I think we cannot say anything about the progress of the project, being outside it. So why not follow in the meantime the last thing that IUPAC endorsed in 1988, and put in their 1990 Red Book: group 3 as Sc, Y, Lu, and Lr? Double sharp (talk) 02:25, 3 August 2020 (UTC)
- @Michael D. Turnbull and Sandbh: Forgot to ping you, sorry. Double sharp (talk) 02:41, 3 August 2020 (UTC)
Contribution #2, Sandbh
Endorsement
- Fluck’s saying that group 3 is Sc-Y-Lu-Lr was a personal opinion, relying on questionable sources.
- Eric seeing Fluck’s statement is a personal opinion, not connected with his role as the Chair of the IUPAC Group 3 project.
- Fluck referring to this form of table being in the new edition of the IUPAC Red Book referred only to the new group numbering scheme.
- The 32 column table appearing in the 1990 Red Book came with a caveat that IUPAC does not advocate any form of table.
- IUPAC is looking at the group 3 question in response to Eric’s request to be able to do so.
Sources in the literature
- The IUPAC survey spanned the period from the 1970s to the 2010s. You have to remember that the chemistry establishment includes chemists who studied in the 1970s or earlier. The project did not therefore confine its survey to the 2010s.
- There is no profit in deciding we are in a position to decide the reliability of close to 200 textbooks.
- The fact that when Jensen spoke to some chemists and found that they were not aware of any controversy around group is because, effectively, there wasn’t any.
- As Scerri has observed, Jensen was too selective in his arguments.
- Landau & Ligshitz, as noted, IMO, cannot be relied on. On the one hand, Double sharp refers to them as not an important thing. On the other hand, he refers to it "as a world-renowned textbook that got its authors the Lenin Prize of 1962." You can now find my commentary about L&L at the Internet Database of Periodic Tables, run by Dr Mark Leach.
Analysis of sources
Yes, Merz and Ulmer, for example, is not worth considering. They said La does not have a typical d-block-like structure for its conduction band. Never mind non-typical conduction band structures can be found elsewhere (Ca-Ba; Group 11; Bi). Never mind x-ray isochromats of Gd to Lu do not support Merz and Ulmer's conclusion that Lu is more favourably placed in group 3. In fact, over half the lanthanides–not just lutetium—have conduction band structures that are more characteristic of transition metals such as hafnium. Double sharp knows this.
What reliable sources say
- The fact that all of five sources say gas phase configurations should be used with caution (I agree!) and that they are irrelevant, is irrelevant compared to the thousands of sources that use them. Even Scerri (pers. comm.) has noted that gas phase configurations are a highly successful model.
- Yes, indeed "reliable sources usually organise the elements on the basis of blocks", and they often do not even bother to define what a block is. That is because it is self-evident.
- The few sources that question the relevance of the delayed appearance of the first electron are noise, compared to the thousands of textbooks that treat this as non-controversial and show La under Y.
Writing problems
- Periodicity is not based on the Madelung Rule. Periodicity is approximate only. The MR is an approximation of the approximate periodicity seen among the elements.
- Worrying about the MR is an example of what Schwerdtfeger, Smits & Pyykkö (2020) refer to as an unnecessary dispute.
- No, Scerri does not necessarily know what IUAPC has or has not done.
The 2019 article by Scerri
You are reading things into this article when you do not have sufficient awareness of the background to it. --- Sandbh (talk) 07:11, 3 August 2020 (UTC)
Response #3, DS
Unfortunately, Sandbh's above response continues to show him engaging in his previous behaviour: using WP:OR to discredit reliable sources, both by misunderstandings of the sources and using them only selectively.
- IUPAC's endorsement
Sandbh claims that I am reading things into Scerri's 2019 article and comment when I supposedly do not have sufficient awareness of the background to it. This is a rather weird statement considering that I simply reported what he said. Which is, again, as follows:
| “ | Thirdly, I should also mention that figure 3 that I call an optimal table, was already endorsed in an earlier IUPAC report, E. Fluck, New Notations in the Periodic Table, Pure and Applied Chemistry, 60, 3, 431-436, 1988. | ” |
| — Eric Scerri | ||
From this, I do not see how my conclusion "Eric Scerri says that the 1988 IUPAC report was an endorsement of Lu under Y" could be considered as "reading things into this article". It is literally right there in the author's own comment to it.
And even though I quoted Scerri complaining about politics vs science, I specifically said after the quote: "That's why I think we cannot say anything about the progress of the project, being outside it". That seems rather the opposite of reading things into the article. It is rather a case of me refusing to do so.
Now, let us examine Sandbh's statement that I do not have sufficient awareness of the background to it. This is correct, of course. I don't know anything about the background as I am not on the current IUPAC project. But that is also, of course, totally irrelevant for Wikipedia. As shown above, I have read nothing into it when it comes to the crux of whether IUPAC endorsed the Lu form in 1988 or not. Scerri writes that it did, and I simply reported that. The 1988 IUPAC report goes so far as to centre "Sc, Y, Lu, Lr" when it says the scandium group should have that composition (it refers to electron configurations as well as physical and chemical properties to justify why). Why should the background be important for Wikipedia? It is not in the reliable sources. Any attempt to figure out what is not written is original research.
And what of Eric Scerri himself? His article is indeed not being written by him in his capacity of the Chair of the IUPAC Group 3 project. But by virtue of the fact that he is on that project, he seems better placed than any of us editors here as to know what IUPAC has or has not said in the past. Why should Sandbh's opinion have more weight than what Scerri writes regarding whether Fluck's article was a IUPAC endorsement or not?
That IUPAC notes in the 1990 Red Book that it isn't advocating any form is not relevant. Scerri referred to the 1988 report, not the 1990 Red Book, as the endorsement. It is only a confirmation as the 1988 report says "This form of the periodic table will therefore be set out in the Appendix to the new edition of the IUPAC Red Book (Nomenclature of Inorganic Chemistry), but will be related to those alternatives used most widely in the literature." And indeed it does appear there in 32-column form.
- Reading things into sources
Indeed, it is Sandbh who continually in this discussion reads things into sources in order to discredit those that don't show a La table. As one example, he says above "Google image searches are plagued by bias arising from the false impression that the IUPAC *-** table is “official”, and unreliable sources." But the periodic tables found on such a Google Image search generally do not tell us why they decided to show group 3 the way they did. Sandbh reads a reason into them for it that is not there, with no reliable source backing him. Whereas, when I pointed out that sources from the 1970s are by definition going to be outdated because Jensen's 1982 article postdated the 1970s, he simply says that "the chemistry establishment includes chemists who studied in the 1970s or earlier". Then what about that old idea that hypervalent molecules like PCl5 have d-orbital involvement in phosphorus? Many old textbooks will tell you that. Of course we know by now it's not true. Does the old and incorrect understanding still count as part of the "chemistry establishment" even so? Are those articles refuting the idea of expanded octets with d orbitals noise compared to the ever-present textbook error that such things exist? How far do we go back? Nascent hydrogen? Phlogiston?
The bottom line is: before Jensen wrote his article in 1982, most chemists did not know about this issue. That is not my own analysis, it is what Jensen says himself. I did not read that into him; it was right there, and I quoted it above. So why refer to chemistry textbooks before 1982? With a few exceptions, they likely won't reflect what chemists thought about the group 3 dispute at all because at the time it was not on the radar of most chemists.
Of course that does not mean that "effectively, there wasn’t any" dispute, as Sandbh would have it. There was a lot. Bury (1921). Shemyakin (1932). Landau and Lifshitz (1958). Seel (1961 and 1969). Hamilton and Jensen (1963). Hamilton (1965). Matthias et al. (1967). Merz and Ulmer (1967). Chistyakov (1968 and 1970). Luder (1967 and 1970). Wittig (1973). And I got those all just from the reference lists of the IUPAC endorsement of 1988 and the Jensen article of 1982. It just did not flash on the radar of most chemists, maybe because many of these were physicists. If we push back even further there are even the tables of Bassett (1892) and Werner (1905) which would qualify as Lu under Y if not for the tiny problem that Lu had not even been discovered(!).
Above and below I showed and will show how Sandbh tries to discredit two of these (Landau and Lifshitz 1958, and Merz and Ulmer 1967), not through referring to other reliable sources to refute them as outdated, but rather by means of original research and misrepresenting the source.
- Selective usage of sources
Sandbh moreover treats sources with great selectivity, quoting an author when he says something that seems to support the La form, but not when he seems to support anything else. For example: he mentions that Scerri and Parsons criticised Jensen's 1982 article as being too selective in his arguments for Lu under Y. But he fails to mention that Scerri and Parsons, in an article in Mendeleev to Oganesson where these criticisms appear, support Jensen's conclusion of Lu under Y through other arguments. And while he is willing to quote Scerri when it comes to discrediting Jensen's arguments, he tries to discredit Scerri's statement that IUPAC endorsed the Lu form in 1988 as Scerri's personal opinion only.
How can we square Sandbh's statement "There is no profit in deciding we are in a position to decide the reliability of close to 200 textbooks" with his deciding that he is in a position to decide the reliability of Landau and Lifshitz, and Merz and Ulmer?
- Landau and Lifshitz
Now, let me address the point about Landau and Lifshitz. What I said about Landau and Lifshitz was:
| “ | An example of this is where Sandbh refers to his own analysis at Wikipedia talk:WikiProject Elements/Archive 48#Landau & Ligshitz (1958): Redux to justify his contention that Landau and Lifshitz's Course of Theoretical Physics is not a reliable source regarding the matter. That is brought in despite the fact that it is not the important thing here (it's just another source); it is rather just a source that the IUPAC 1988 report cited. And even if it was the important thing, it remains that this is a world-renowned textbook that got its authors the Lenin Prize of 1962. | ” |
Okay, rereading it now, indeed this was unclear. So let me clarify its meaning. Here is what I meant, expressed more discursively.
First of all Sandbh's attempt to discredit its reliability is misplaced, because the important source that is really the crux of why I am proposing this is that IUPAC endorsed the Lu form in 1988. Landau and Lifshitz merely gain some peripheral relevance because they are one of the sources that IUPAC cited to justify its endorsement.
And second of all, even if Landau and Lifshitz were the crux of the matter, Sandbh's attempt to discredit its reliability would become rather astonishing. This is not any old book. It is a book called "renowned" in Science and "celebrated" in American Scientist. As I mentioned: it won its authors the Lenin Prize of 1962, the first time that was ever awarded for teaching physics. In short: this is a classic text. IUPAC has interpreted it as supporting group 3 as containing the elements Sc, Y, Lu, and Lr in its endorsement of 1988. What is Sandbh doing trying to discredit its reliability? And, most importantly, what is Sandbh doing by referring to a thread in which he purports to do that, but in the process draws a so-called "Landau and Lifshitz periodic table" when Landau and Lifshitz did not give a periodic table in their discussion? And when that "Landau and Lifshitz periodic table" is extended by Sandbh beyond curium, the last element Landau and Lifshitz actually gave a configuration for, thus necessitating Sandbh to read into Landau and Lifshitz's minds to figure out how they would deal with the unprecedented anomalous electron configuration of lawrencium ([Rn]5f146d07s27p1 rather than [Rn]5f146d17s2)? Not to mention that it would require more than just mind-reading, since this anomalous electron configuration was first predicted in 1971 when Landau had already passed away, and it only got clear experimental support in 2015 when Lifshitz had also already passed away?
And what does Sandbh say above? "Landau & Ligshitz [sic], as noted, IMO, cannot be relied on." In his opinion? Why is his opinion more reliable than the fact that this is a standard text and how IUPAC saw it?
- Merz and Ulmer
Sandbh likewise uses his own WP:OR to dismiss Merz and Ulmer as "not worth considering". Despite the fact, of course, that they got published in an academic journal and are certainly a reliable source on those grounds. And that Jensen cited them and included their argument in his 1982 article. And that the IUPAC report, which despite what Sandbh says is a report and not just Fluck's personal opinion, cited Jensen's article as justification for its endorsement of Lu under Y. But to Sandbh that is all irrelevant because his original research claims it doesn't work. Not to mention that his original research irrelevantly brings in the lanthanides from Gd to Yb inclusive, forgetting that none of them are actual candidates for being placed under yttrium: what is relevant is only La or Lu. And finally he claims I know this. Why? Because when he and I agreed on La in the past we mentioned that in that old 2017 submission to the IUPAC group? Then what about me having explicitly said that I have since learnt more and withdraw my support for it?
- Wikipedia policy
Is any of this behaviour of Sandbh in accordance with the Wikipedia policies of WP:RS, WP:NOR, and WP:NPOV? Sources, if reliable, should not be selectively quoted to support an editor's bias. And no amount of original research will discredit a source for Wikipedia. Noting that a source both supports a statement and its opposite might do the trick, noting that more recent reliable sources say something else would too, but coming up with a new and original counterargument will not do.
I do not propose to do that at all. Indeed, I have mentioned above that even though I would prefer to describe Madelung's rule following the Lu table due to its concordance with it (Lu-Lr), I would add a note or even a full-blown extra paragraph detailing that many authors use a La table instead. And then discuss it appropriately using reliable sources. Rather than sweep inconvenient facts under the rug without a good reason, such as a later, very reliable source saying "actually this view is obsolete".
- Gas-phase ground-state electronic configurations
Sandbh refers to the fact that many sources do still continue to give gas-phase ground-state electronic configurations. Indeed, they do. But what consequences are drawn from this? Nothing without contradiction, so far as I can see.
Let us consider what Greenwood and Earnshaw says about such anomalies. Let us take for example the nickel group, because it has a notable total mismatch in such configurations. Nickel is d8s2, palladium is d10s0, and platinum is d9s1. Greenwood and Earnshaw show these in their table on p. 1148. And what conclusions do they draw from it? Absolutely nothing. They don't even mention it in the main text. Rather on p. 1150 they list complexes, including neutral ones. In which, corroborating my point, [Pt(PPh3)3] is given as an example of a Pt(0) complex and assigned to d10, which is not the configuration of a bare Pt atom.
Now, Greenwood and Earnshaw do consider it for the copper group. On p. 1176 Greenwood and Earnshaw say about the stable members of the Cu group "This is the first triad since Ti, Zr, and Hf in which the ground-state electronic configuration of the free atoms is the same for the outer electrons of all three elements". Except that that's an inaccurate statement because Mn, Tc, and Re are another such triad. It's just that they don't think it is because in their tables Tc is claimed to be d6s1, while Mn and Re are claimed to be d5s2 (p. 1043). But then again their statement is still inaccurate even from the data they used because on p. 979 they claim that V, Nb, and Ta are all d3s2, when Nb is now known to actually have a d4s1 ground state. No one can blame them about that, as the lowest J-level of the d3s2 configuration is literally only 0.14 eV up from the ground state. So their observation is not even true on two levels: it is contradicted by the data they themselves use, and the data they use has been superseded by better data since, but the better data also contradicts their observation. Not much analysis is needed for that, it is an obvious contradiction with publicly accessible data. Unlike what Sandbh has to do to discredit Merz and Ulmer.
Now, do they draw any consequences from this tidbit? They try once, where they claim on p. 1177 that the physical properties of the coinage metals Cu, Ag, and Au can be related to the d10s1 configuration. But that is refuted by the Fernandes et al. article I cited above:
| “ | The elements of the coinage family have an electronic configuration given by (n-1)d10ns1 and are said to have irregular electronic structure (with respect to the electronic structure and orbital ordering based on the hydrogen atom). This means that, for this family, the reference line lies in the s orbital while the d orbital should be lower in energy and narrow since all d components are doubly occupied (as shown in Figure 1). Given that picture, these atoms should present a metallic band similar to that of an alkali metal when forming a solid, i.e., each atom contributes with one electron to the chemical bond, which is constructed with s and p orbitals and thus forms an sp band with relatively low occupancy. The d orbitals of each atom are completely filled and, therefore, should not directly participate in the bonding, only providing certain freedom to the external electron in the s orbital. This would lead to a metal which should have a relatively high electron conductivity, but also very reactive since the binding energy involves essentially one electron per atom.
Obviously, this is not an accurate description of copper, silver and gold solids which have characteristics of noble metals and are among the most conducting metals. Then what went wrong in the analysis we used? Maybe it is too simple to capture the qualitative behavior of the chemical bond or, maybe, it is missing some information. At least, in this case, the analysis is missing a very important piece of information: the transition metal elements are not adequately represented by a single electronic configuration. This happens in atoms and other systems in which energy levels are close to each other or when two different configurations lead to the same energy. This situation arises in transition metal atoms due to relatively close d and s orbital energies. Each electronic state of a given transition metal atom is probably not well represented by a single configuration, but instead, it is a combination of two or more configurations. Let’s take the coinage metals as an example: the copper electronic ground state is not best represented by 3d104s1. A better representation of such an electronic state would be 90%(3d104s1) + 10%(3d94s2) (obtained with the solution of the non-relativistic Schrödinger equation under the MRCI/aug-cc-pwCV5Z approximation). This means that bonds involving copper atoms should present characteristics of both configurations. The first one was described above: it leads to an alkali-like metal with a high mobility electron per atom. We shall now analyze the latter configuration, 3d94s2. This leads to a very different bond scenario: the reference line is at the 3d orbital, i.e. above the 4s and thus the sp band can accommodate more electrons. Besides that, one of the d orbital components participates directly in the bonding, creating a slightly broader d band which would be occupied below the reference line (one unpaired d electron per atom). If the former configuration can be related to the alkali metal, the latter is to some extent related to the alkaline earth metal, since it has two electrons in an s orbital that will populate an sp band. However, the binding electrons from the d band can flow to the sp band and increase the binding energy. In this case, the electrons in both the sp and d bands would enhance the chemical bond and also the number of electrons available for conduction. This means that the solid formed by this configuration would have much more binding energy than an alkaline earth metal (considering the same period of the periodic table) with a comparable or higher conductivity due to its three electrons per atom. Combining the solids that would result from both 3d104s1 and 3d94s2 configurations isolated, one has a more complete picture of the characteristics of copper: a high conducting metal (from 3d104s1) with high binding energy (from 3d94s2). Since the latter contributes only with 10% of the atom behavior, the characteristics of this configuration are attenuate in the resulting solid, making it only regular in its binding energy and mechanical properties when compared to other transition metals, but sufficient to reduce its reactivity that would result from a low binding energy per atom. Another effect that would increase the reactivity is the excess of binding sites unoccupied, but this is not an issue for this system. |
” |
| — G. F. S. Fernandes, L. A. Cunha, F. B. C. Machado, and L. F. A. Ferrão, The Chemical Bond across the Periodic Table (2020) | ||
It's not just my original research saying "let's supersede this source because I have my own argument against it, even though that never appeared in the literature". I refer to a later source that refutes this one. Not to mention that Greenwood and Earnshaw themselves weaken their own statement on p. 1177 itself in the footnotes by themselves referring to the sp conduction band to explain the colours, which rather contradicts the idea that things come from a d10s1 configuration with no p orbitals involved.
And indeed, when explaining the valencies of the coinage metals on p. 1180, they take care to focus on ionisation energies and hydration enthalpies, rather than attempt to derive things from gas-phase ground-state electronic configurations. Indeed, they take care to note that CuII is more stable than CuI in aqueous solution despite the latter's d10 configuration.
So, where is the relevance? When reliable sources just put the configurations in tables, very rarely actually refer to them, and the one time they do refer to them proceed to contradict themselves? And when the instance that is being referred to is exactly an instance refuted by later sources with an explanation?
And is a personal communication from Scerri a relevant source for Wikipedia, considering that no one here can verify it?
- Differentiating electrons
And that's not even getting into the murky notion of differentiating electrons. Which occurs precisely twice in Cotton and Wilkinson and does not even appear in the index of Greenwood and Earnshaw. And for which I have never even seen any reliable source clearly defining them across the whole periodic table. Where does any reliable source define what the differentiating electron between vanadium 3d34s2 and chromium 3d54s1 might be?
If the La form is based on ground-state gas-phase configurations and differentiating electrons, then this evidently shows rather that its bases are totally at odds with what reliable sources say about the elements outside the main groups. Faced with a choice between (1) following La under Y with a plurality of textbooks only that vanishes once you look at periodic tables in other places, whose bases are contradicted by reliable sources, and (2) following Lu under Y which was endorsed by IUPAC in 1988 and whose bases are consistent with reliable sources, (2) is clearly better.
As for the delayed start of La under Y, Sandbh refers to "thousands of textbooks" when the IUPAC survey shows only 130 such. There are actually a few interesting questions about this that it would be good to know the answer to. How many such textbooks give the standard Madelung rule with 4f before 5d (and if so how do they deal with the contradiction?), and how many edit it to claim that one 5d electron hangs up before 4f is filled (yes, that's sometimes done, see the 2006 edition of Zumdahl and Zumdahl's Chemistry)? And how many note the contradiction that such an edit makes with the general statement of the rule: "electrons fill in orbitals with increasing n+l; if two orbitals have the same value of n+l, the one with the lower value of n fills first"?
Why should we be forced to leave a gaping contradiction or go off on a tangent when explaining the Madelung rule on Wikipedia, when we could just solve all these problems by taking the form that IUPAC endorsed back in 1988 as a default? It even makes complete sense since we are doing this as an interim measure before IUPAC's current project makes a new recommendation.
- Blocks
What a block is is not "self-evident", despite what Sandbh says. The fact that this debate has lasted nearly a century and has at stake whether lanthanum is an f-block or d-block element is rather self-evident proof that it is not that obvious. Sandbh has a rule for block start indeed. According to Wikipedia talk:WikiProject Elements/Archive 42, it is "a block starts upon the first appearance of the applicable electron" (must be in the gas-phase ground-state, or else La goes straight to the f-block). I am not aware of this appearing in any reliable source anywhere. (It comes from that submission I made to IUPAC with him when I supported La, but again: I withdrew my support for it when I learnt more.) And I and Droog Andrey have been questioning this definition greatly in that very archive. How can Sandbh still claim, after all that, that it is "self-evident"?
Indeed, reliable sources often refer to blocks. But where is the reliable source defining them that does not contradict its own definition?
- Periodicity and the Madelung rule
Let us see what the IUPAC Commission on the Nomenclature of Inorganic Chemistry had to say on the matter.
| “ | On August 19, 1987 CNIC heard delegates from the Deutsche Zentralausschuß für Chemie, the German National Adhering Organization to IUPAC, and the American Chemical Society on this matter. Prof. Brodersen, University of Erlangen, Germany, recommended the use of the "code of chemical elements" (ref. 14) as the periodic system. CNIC rejected the proposal arguing that 1) the code of elements is rather an illustration of the Aufbau principle from which a periodic system is to be developed... | ” |
| — E. Fluck, New notations in the periodic table (1988) | ||
That's rather in contradiction with Sandbh's statement "Periodicity is not based on the Madelung Rule".
The periodic table comes from the Aufbau principle, because chemical periodicity is based on electronic structure, and the Aufbau principle predicts that. Of course, for d and f elements what is more important is the total number of valence electrons and their valence orbitals, rather than minutiae of exact ground-state gas-phase configurations. That's why you can see that anomalous configurations often make precisely no difference to the chemistry of the d and f elements concerned. That is the understanding of the recent reliable sources focusing on the matter.
- Conclusion
Sandbh's selective usage of and misreading of sources is something that he already did in that long, long four-archive discussion at Wikipedia talk:WikiProject Elements. I mentioned that he was taking sources out of context, Droog Andrey (an actual chemist) also noted that some of his sources did not support what he was using them for. It is, in fact, a significant part of why I eventually lost my patience with Sandbh.
Now, I did go too far above: I apologised, we went back to a gentlemen's disagreement. I would like to keep it as a gentlemen's disagreement, as we have fruitfully collaborated on Wikipedia before. And so far it has been simply discussion. But now the content of the article is at stake regarding which form should be taken as a default. And if Sandbh should continue to in the above way favour his own original research over explicit statements by reliable sources when it comes to the content of the article, then I am sorry to say that I will have no choice but to reluctantly escalate this dispute to WP:ANI. Double sharp (talk) 09:55, 3 August 2020 (UTC)
- @Michael D. Turnbull, Sandbh, and YBG: Forgotten pings again, my apologies. Double sharp (talk) 09:55, 3 August 2020 (UTC)
Contribution #3 by Sandbh
| Round | DS post | Sandbh response |
|---|---|---|
| 1 | 2,050 | 620 |
| 2 | 3,830 | 590 |
| 3 | 4,385 |
It's unfortunate this topic takes up so much wordage. Addressing Double sharp's contributions now takes me so long it's starting to impact my RL obligations. The table shows the cause.
When I can I'll see if I can give some concise observations.
Double sharp and I, as he said, have worked together productively on other matters. No doubt we will continue to do so. And I look forward to it.
His threat to take this particular matter to WP:ANI leaves me speechless. --- Sandbh (talk) 01:00, 4 August 2020 (UTC)
- @Sandbh: As I see absolutely nothing in your posts that addresses your misuse of sources, I will now be forced to act on the threat. Double sharp (talk) 01:24, 4 August 2020 (UTC)
ANI thread
Please see WP:ANI#Misuse of sources by User:Sandbh (started by me). Double sharp (talk) 02:09, 4 August 2020 (UTC)
