Talk:Acid dissociation constant/Archive 3
| This is an archive of past discussions about Acid dissociation constant. Do not edit the contents of this page. If you wish to start a new discussion or revive an old one, please do so on the current talk page. |
| Archive 1 | Archive 2 | Archive 3 | Archive 4 |
Equilibrium constant
There are a couple of very minor problems in the definition of an equilibrium constant. Firstly, I personally have never seen the abbreviation Kt for the thermodynamic equilibrium constant. From International Union of Pure and Applied Chemistry (1993). Quantities, Units and Symbols in Physical Chemistry, 2nd edition, Oxford: Blackwell Science. ISBN 0-632-03583-8. p. 50. Electronic version., "ISO and the IUPAC Thermodynamics Commission recommend the symbol Ko and the name 'standard equilibrium constant', but some thermodynamicists prefer the symbol K and the name 'thermodynamic equilibrium constant'."
- As you say, there is no consensus for this notation. In the present context I think that the important thing is to differentiate clearly between a constant based on activities and one based on concentrations, but I don't like either K
oor K. What do you suggest?Petergans (talk) 15:23, 22 November 2008 (UTC)
Secondly, activities (and therefore Ko) are indeed dimensionless, because they are defined by dividing by the standard state: ie a(H+) = c(H+)γ(H+)/co. In standard notation, the first two equations should be:
Only in this way can you keep Ko as a dimensionless quantity suitable for taking a logarithm thereof. Physchim62 (talk) 12:38, 22 November 2008 (UTC)
- This is a valid point of view, but I have taken another, equally valid one, namely, that the standard state is defined by setting activity to one. On your system the activity coefficient is dimensionless, on mine it has the dimension of inverse concentration. I have tried to simplify this section as much as possible for the sake of the general reader. Otherwise we have to get into a detailed discussion of activity and standard states, which I suggest is too complicated for this article. I have tried to improve the article activity coefficient to reflect this issue. Maybe that is the place where the detailed discussion should go? As mentioned in that article, when the concentrations are expressed as mole fractions the question of dimension does not arise. That's why I have side-stepped the issue. I've also side-stepped the question of molar and molal concentrations. Petergans (talk) 15:23, 22 November 2008 (UTC)
- Hmm, that's not actually the definition of standard state, or of activity coefficient… I'm looking to do a minor blitz on standard state, so I'll take a look at activity coefficient as well! Might it not be simply to start out by saying: "The acid dissociation constant is the equilibrium constant for the reaction HA ⇌ H+ + A−." That definition has two main advantages: it is independent of the solvent used, and it closely mirrors the way Ka values are measured in practice. It's disadvantages are that it appears to give a quantity with dimensions of concentration (a common problem for equilibrium constants) and it does not mirror the actually Brønsted–Lowry equilibrium, which would be HA + S ⇌ HS+ + A−. Those advantages and disadvantages could be easily mentioned, so long as we point out that the conventional definition really is Ka = [H+][A−]/[HA], this isn't just some simplification we've made up! Physchim62 (talk) 13:22, 23 November 2008 (UTC)
I am following a long-established convention. For example Rossotti & Rossotti (1961), equation 2-1 (mass-balance) reads
implying that concentration = activity / activity coefficient. Regarding the definition of Ka, my objective has been to show what approximations are involved when the simplified formula is used, because sometimes they can come back to bite the unwary. Petergans (talk) 16:46, 23 November 2008 (UTC)
- The long-established convention is that Ka = [H+][A−]/[HA], or, as IUPAC prefers these days (2006 Technical Report, to give a dimensionless Ka), Ka = [H+][A−]/[HA]c
o[1]. It is the thermodynamic dissociation constants which are "unconventional", and this indeed causes some confusion (eg, at strong acid). Physchim62 (talk) 20:03, 23 November 2008 (UTC) - The current definition of the Henry's law activity coefficient based on amount concentrations (from the IUPAC Green Book) is ac,B = γc,BcB/c
o. This gives dimensionless activity coefficients (as the term "coefficient" would imply), as well as dimensionless activities (as has long been conventional). Physchim62 (talk) 20:30, 23 November 2008 (UTC)- It appears that the confusion arises from IUPAC having changed its recommendations. I have a copy of the Compendium of Analytical Nomenclature 2nd. edition (1987) which recommends the symbol y, not γ for activity coefficient. Can we anyway agree that the current text is sufficient for its purpose, which is to emphasise the fact that when K defined as a concentration quotient it is implicit that Γ is a constant?
- The reference [2] is very useful and I will add it in the appropriate place. Petergans (talk) 10:16, 24 November 2008 (UTC)
- I had a go at rewriting this section, and I agree that I'm not really adding anything important beyond what is already there. The IUPAC report is the only IUPAC recommendation I've been able to find which risks a definition of Ka: luckily it agrees with the article! IUPAC has certainly changed its definitions in the past: I've found a 1976 recommendation which has activity coefficients defined as you define them above, and the symbolism changed with the approval of ISO 31. The 3rd edition of the "Orange Book" uses γ for the activity coefficient (see section 3.3). Physchim62 (talk) 13:54, 24 November 2008 (UTC)
- I've tried modifying your sandbox, but am not convinced that the result is any better than the current text. It is a little more rigorous at the expense of being more difficult to read. I'd like to keep the current text in spite of the fact that it is out of step with current IUPAC recommendations, because it is simple, clear and logical. Petergans (talk) 16:36, 24 November 2008 (UTC)
- The alt text is good if a bit too descriptive in places. Ideally it shouldn't give any more information than the picture or equation. So I don't know why we should note that H2O is water, or the gamma is a Greek letter, in the alt text; this information is not available in the graphics or the equations. Graham87 06:06, 26 November 2008 (UTC)
- I haven't completed a review of the alt text, but I agree, it should only say what the image or equation says. Petergans (talk) 08:37, 26 November 2008 (UTC)
Tim's inadvertant errors
Having criticized User:TimVickers for this set of edits – made entirely in good faith and with the objective of improving the lead – intellectual honesty requires me to be specific, to see if editors can get ideas for improving the understandability of the article. IMHO:—
- Ka is not a physical constant in the sense of the speed of light or the rest mass of an electron. Ka are fairly constant, constant enough to be useful but variable enough to catch the unwary. K
ovalues are only "constants" because they include experimentally-determined 'fiddle-factors' (better known as activity coefficients). - [minor point] I'm not sure if Ka "measures" or "describes" the strength of an acid. Similarly for bases, where the phrasing grated even more for me.
- All solvents will allow acid–base chemistry to occur. Believe me, I've done acid–base chemistry in hexane, usually using butyllithium as the base and sometimes using toluene as the acid (great for safely cleaning your syringes!). The pKa of hexane is estimated to be around 45, although it can't be determined (see below).
- Ka values are never measured, they are determined: that means they are calculated on the basis of a number of other measurements. If pKa falls between 2 and 11, I suppose you could, just about, determine it with a single measurement: in practce, this is never done. Moral: there is a difference between measurement and determination.
- You can investigate the formation of complexes without knowing anything about pKa values, I've had several students who've done it, at least one of whom has published papers on their results. The trick is to make your (neutral) complexes in methanol, using triethylamine to mop up any excess hydrogen ions (which should never really be called "protons" in a condensed phase, even if we all do it). So I wouldn't really call that knowledge "vital". On the other hand, if The Boss tells you to transfer your nice reaction in methanol to 6 M aqueous nitric acid then you need to think very seriously about pKa values! (not my personal experience, but a real one all the same) That is the difference between a "quantitative understanding" and a mere "investigation".
- [additional point] Ligands are, almost by definition, bases: there is one well-known example of a ligand which is a Lewis base without being a Brønsted–Lowry base, but I feel that it is the exception that proves the rule! However this particular misphrasing was there before Tim's edits, and I include it here merely for completeness.
Physchim62 (talk) 00:25, 27 November 2008 (UTC)
- While one can make a distinction between measurement and determination, I think you are being too rigid with respect to this point. Perhaps most things in science are not really "measured" directly, and yet scientists write all the time about measuring things that are arguably determined. Just to give one example look at the title of this IUPAC report: The Measurement of pH - Definition, Standards and Procedures. Yet one does not measure pH directly but determines it based on other measurements, theories, and conventions, even if all the logic is hidden inside an easy-to-use pH meter. --Itub (talk) 10:11, 7 December 2008 (UTC)
Parentheses
Collins English dictionary: Parenthesis A phrase, often explanatory or qualifying, inserted into a passage with which it is not grammatically connected. In "A generic acid, HA, .." HA is grammatically connected. It is neither explanatory nor qualifying. It defines the symbol to be used for a generic acid in the equilibrium expression which follows. Petergans (talk) 11:54, 29 November 2008 (UTC)
Acid strength
Im sorry chaps, the sentence " A high value indicates that an acid is stronger, because the acid produces more hydrogen ions in solution" is incorrect. From pH#Calculation of pH for weak and strong acids
- pH = ½( pKa - log c)
where c is the acid concentration. This equation clearly indicates that the pH of a solution of a weak acid depends on the acid's concentration, which is obvious when you think about it. The ICE table calculation implies it, too. That's why I wrote, some time ago, "at a given concentration".
The introduction of corrosiveness in the lead is not right, because no mention of it is made in the body of the article.
Where on earth did the phrase "because they tend towards infinity" come from? I don't understand the phrase or what it is meant to convey. What has infinity got to do with thermodynamic quantites?
There are not changes for the better. Petergans (talk) 14:31, 2 December 2008 (UTC)
Further material
During the helter-skelter of the last few days I did not have time to include any of the following material in the applications section. It was sent to me by Ivo Leito after some e-mail correspondence between us. I will not incorporate them myself, for the reasons given in the next section. I hope someone else will do so.
- Organic synthesis
- pKa values of acids and bases are very important in organic synthesis. Many of synthetically useful reactions proceed via acid anions (most notably carbanions – anions of CH acids). Such reactions are catalyzed by bases that generate the anions by deprotonating the corresponding acids. In order to generate a sufficient amount of the anion the pKa value of the catalyst-base has to be of the same order of magnitude or higher than the pKa value of the acid, which is to be deprotonated. At the same time at too high basicity of the catalyst competing side-reactions may occur – different other compounds in the solution may be deprotonated and the generated anions will compete with the desired anions. Mostly these reactions are carried out in nonaqueous solvents and the pKa values of relevance are those determined in the corresponding solvents.
- Reference: March's Advanced Organic Chemistry: Reactions, Mechanisms, and Structure, 6th Edition, Michael B. Smith, Jerry March, Wiley, 2007.
- Liquid chromatography
- pKa values of acids and bases are very important in liquid chromatography. In reversed-phase partition chromatography – the most common mode of analytical liquid chromatography– usually polar buffered water-organic mobile phases and nonpolar stationary phases are used. In such system nonpolar molecules are retained more strongly than polar ones. Partition properties of the analyzed substances depend strongly on their ionization states. Neutral molecules are less polar than their ionized counterparts and are thus more strongly retained by the stationary phase. Therefore neutral forms of acids and bases are retained more strongly than their ionized forms. Mobile phase pH enables to control the extent of protonation-deprotonation of acids and bases and varying the pH allows to vary retention times of acids and bases and provides an additional possibility for optimizing chromatographic separations.
- Reference: Practical HPLC Method Development, 2nd edition, Lloyd R. Snyder, Joseph J. Kirkland, Joseph L. Glajch, Wiley, 1997.
Petergans (talk) 18:50, 7 December 2008 (UTC)
FAC not promoted
I am truly sorry that it has come to this after all the time and effort that has been put in, not only to writing the article, but also to replying to the concerns expressed at FAC. I offer my sincere thanks to all who have participated.
It appears that consensus is impossible between us professionals and those reviewers at FAC who, notwithstanding their ignorance of the subject matter, are prepared to instruct us on how to present our material. For this reason I do not intend to spend any more time on this article and do not intend to re-submit as FAC. Petergans (talk) 11:03, 7 December 2008 (UTC)
- I'm very sorry as well, not least because of your disappointment. I too honor the tremendous work that you and others have lavished on the article thus far, and I hope that all of us can do a better job in the future at forestalling such disappointments.
- I urge you to consider, however, a strength of Wikipedia: that knowledgeable authors can get feedback — sometimes painfully blunt or poorly informed feedback — from their potential readers on the intelligibility of their prose. We don't write for ourselves, but to help others understand and appreciate new things. True, there's no pleasing everyone; but neither should we ignore good-faith criticisms, right?
- I have not forgotten my pledge to help with the article, and I suspect several others will be committed to improving it as well. There are many paths by which we can reach our common goals; perhaps a fresh path is called for? You may like what you see, once we've made some progress, and I hope you'll consider rejoining us. Proteins (talk) 11:51, 7 December 2008 (UTC)
Fair enough. I feel that I've given all that I can give and it's time for others to pick up the baton. You mentioned Linus as a model. Well he was a genius and if I had a fraction his talent I, too, would be able to write like an angel :-). BTW I still have the EXCEL files from which some of the diagrams were made. The problem with the pictures appears to be associated with the reduction to thumbnail size as the originals do not look so poor (example at the right). Indeed, HySS (freeware) was designed to produce diagrams of publication quality at the width of half a JACS column. Petergans (talk) 14:11, 7 December 2008 (UTC)
- I too think that it is a shame that the article was not promoted. However, in the light of aim of Wikipedia (being an anccylopedia) my opinion is the exact opposite of the of Petergans. Yes I see that the reviewers and the topic experts/professionals did not get to an agreement. I think it the aim of the review is twofold. 1) Make sure that the content is relevant and of high quality (this is not what the discussion is about) 2) To make sure the content experts present the content in a way that is understandable and jargon free for the interested intelligent reader with some background knowledge. For this 2nd aim lay-reviewers are not merely equal, but actually better suited than content experts, as content experts may have mistaken ideas about background knowledge. In my view, the reviewers tried their best to get to an agreement how the content presentation maybe improved, which was not taken sufficiently serious by the content experts. This means in my opinion that the decision not to promote was justified as the article was no compelling or even brilliant prose aimed at the moderately knowledgable Wikipedia user. Arnoutf (talk) 17:20, 1 January 2009 (UTC)
- The real dilemma here is that people who know little or nothing about the subject thought that the presentation could be improved without having any idea as to how to do it. The prose may not be "brilliant" but I don't think a better concise presentation of the subject matter can be found anywhere, not in any text-book or monograph or review. We wanted to give present the topic with full scientific rigour, not as a dumbed-down version. This may not appeal to "moderately knowledgeable" people, but my concept of an encyclopaedia is that it covers all knowledge. This means that it has to cover topics that require some previous specialist knowledge and indeed WP does do so, in all the sciences. The pity is that none of those articles is ever likely to make the grade for FAC, for the reasons so clearly expressed above. Petergans (talk) 22:56, 1 January 2009 (UTC)
- I agree it is a very fine line between dumbing down and writing an accessible text. I agree that this is very difficult, and may result in many very good articles never reaching FAC. The difference between very good and featured should count however, and I think that in the science articles this line is guarded very thoroughly. On the upside this also means that science FA are likely to be really worthy of that title (I have seen some sports or computer games articles making FA wich I would rate at B-class myself....). Arnoutf (talk) 10:42, 2 January 2009 (UTC)
- Well, I don't see how anyone can make an objective judgement concerning the quality of something they don't understand. Petergans (talk) 20:04, 2 January 2009 (UTC)
- What I think can be done, for someone who has enough of the prerequisite knowledge to be able to learn from the article, is to judge how well it says what it says (as opposed to judging whether what it says is correct). That still leaves the risk of a non-expert rephrasing something and unwittingly losing accuracy. I think it would be good for those such as myself who are not expert in the topic to propose wording changes here on the talk page, rather than in the article, as it is evident that sometimes those changes have caused unintended changes in the meaning. Mike Christie (talk) 21:50, 2 January 2009 (UTC)
- Well, I don't see how anyone can make an objective judgement concerning the quality of something they don't understand. Petergans (talk) 20:04, 2 January 2009 (UTC)
- I agree it is a very fine line between dumbing down and writing an accessible text. I agree that this is very difficult, and may result in many very good articles never reaching FAC. The difference between very good and featured should count however, and I think that in the science articles this line is guarded very thoroughly. On the upside this also means that science FA are likely to be really worthy of that title (I have seen some sports or computer games articles making FA wich I would rate at B-class myself....). Arnoutf (talk) 10:42, 2 January 2009 (UTC)
- The real dilemma here is that people who know little or nothing about the subject thought that the presentation could be improved without having any idea as to how to do it. The prose may not be "brilliant" but I don't think a better concise presentation of the subject matter can be found anywhere, not in any text-book or monograph or review. We wanted to give present the topic with full scientific rigour, not as a dumbed-down version. This may not appeal to "moderately knowledgeable" people, but my concept of an encyclopaedia is that it covers all knowledge. This means that it has to cover topics that require some previous specialist knowledge and indeed WP does do so, in all the sciences. The pity is that none of those articles is ever likely to make the grade for FAC, for the reasons so clearly expressed above. Petergans (talk) 22:56, 1 January 2009 (UTC)
- I too think that it is a shame that the article was not promoted. However, in the light of aim of Wikipedia (being an anccylopedia) my opinion is the exact opposite of the of Petergans. Yes I see that the reviewers and the topic experts/professionals did not get to an agreement. I think it the aim of the review is twofold. 1) Make sure that the content is relevant and of high quality (this is not what the discussion is about) 2) To make sure the content experts present the content in a way that is understandable and jargon free for the interested intelligent reader with some background knowledge. For this 2nd aim lay-reviewers are not merely equal, but actually better suited than content experts, as content experts may have mistaken ideas about background knowledge. In my view, the reviewers tried their best to get to an agreement how the content presentation maybe improved, which was not taken sufficiently serious by the content experts. This means in my opinion that the decision not to promote was justified as the article was no compelling or even brilliant prose aimed at the moderately knowledgable Wikipedia user. Arnoutf (talk) 17:20, 1 January 2009 (UTC)
Base edits
I hastily attempted to explain the base section without thinking it through. What I have realized will help in explaining the Kb though. After reflection Kb involves two equilibria instead of the simple one that Ka involves, since it not just involves the formation of the conjugate acid (as I was thinking) but the disassociation of water (I realized the mistake in my math was stupidly making the OH times the H equal to water when multiplying by 1/Kw is required to convert the two).
I am suggesting something like this: Kb represents the ratio of the associated acid to water (i.e. If the Ka of an acid is 1.0 x 10-7 then Kb will be 1 x 10-7 (much less associated acid than water) because Kw (which is the Ka of water) is much lower than the Ka of the acid.)--Jorfer (talk) 01:50, 12 December 2008 (UTC)
- Jef, I don't wish to be unkind, and I hope you will take this as constructive comment. The truth is, as shown by your own admission that you had not thought through what you had written, that you are out of your depth here. There is no shame in that. What is shameful is that you won't take on board comments from people like Itub. Regarding pKb all that needs to be said is that the sum of pKb of a base and pKa of its conjugate acid is equal to pKw. Nowadays people usually determine protonation constants so pKb is only of historical interest and does not merit a long discussion.
- Please don't edit stuff you do not thoroughly understand. Petergans (talk) 10:35, 12 December 2008 (UTC)
- OK, I won't make it a long discussion, just a simple explanation. I know that my editing is not perfect but I am confident other editors will hold me accountable. Making mistakes is part of the process of making an article the best it can be.--Jorfer (talk) 21:02, 14 December 2008 (UTC)
- Your edits simply do not make sense - they are not improvements or clarifications. You don't know enough about the subject. Your lack of experience is painfully obvious. Please turn your attention to something you really know about. We have not made up the present text. It is waht you will find in many text-books. Please leave well alone. Petergans (talk) 20:10, 15 December 2008 (UTC)
- I feel my most recent attempt is valid (though I admit that my first attempt was mistaken and my second attempt was not good either). Some explanation is needed, and since no one else has been willing to, I attempted to. I would not have done this if I was not confident it would quickly result in an accurate description through collaboration. I messaged Proteins so he can weigh in.--Jorfer (talk) 01:13, 16 December 2008 (UTC)
- I suggest that when Proteins is free to collaborate with Petergans on the article, that would be a good time for those who are non-experts to pay attention. Towards the end of that process I think the experts would benefit from hearing which parts of the article are easy for non-specialists to understand. I agree that there have been outbreaks of testiness here, but my experience working with Petergans on this article convinces me that a productive collaboration between experts and non-experts is possible. The article does need to be made as clear as it can be to non-specialists. I believe Petergans, Itub and the others are acting completely in good faith when they revert edits which they say are creating errors and inaccuracies. I'll keep the article on my watchlist, and I hope Proteins can find the time to work on it at some point. Mike Christie (talk) 18:57, 4 January 2009 (UTC)
- I concur with Mike. I made a sequence of edits to the article on 19 December addressing some of the concerns raised in my FAC review. These edits have not been reverted. Geometry guy 19:08, 4 January 2009 (UTC)
- I suggest that when Proteins is free to collaborate with Petergans on the article, that would be a good time for those who are non-experts to pay attention. Towards the end of that process I think the experts would benefit from hearing which parts of the article are easy for non-specialists to understand. I agree that there have been outbreaks of testiness here, but my experience working with Petergans on this article convinces me that a productive collaboration between experts and non-experts is possible. The article does need to be made as clear as it can be to non-specialists. I believe Petergans, Itub and the others are acting completely in good faith when they revert edits which they say are creating errors and inaccuracies. I'll keep the article on my watchlist, and I hope Proteins can find the time to work on it at some point. Mike Christie (talk) 18:57, 4 January 2009 (UTC)
Petergans and anonymous IP edits
I want other editors to weigh in on Petergan's removal of wording I included to broaden access, on what seems to me to be his use of an anonymous IP as a hostile sockpuppet, and on his strikethrough edit (all of which can be seen by looking at the history).--Jorfer (talk) 23:52, 2 January 2009 (UTC)
- This is a disgraceful personal attack. I NEVER use an anonymous IP. Petergans (talk) 09:45, 3 January 2009 (UTC)
- Whatever disagreements you may have with Petergans, as far as I have seen he is unfailingly polite and I doubt very much that that hostile IP is him. Regarding the edits themselves, in my opinion he is correct in removing the inaccuracies and misconceptions that you have introduced as a result of your limited understanding of the topic. I suppose that's the reason the IP removed them too, although with a less civil edit summary. --Itub (talk) 17:19, 3 January 2009 (UTC)
- If it wasn't for this edit, I probably wouldn't be accusing him of such. Since IP edits are few and far between on this page, and since the IP is exhibiting extreme anger, it does not appear to be someone detached from the article. These are the first edits this IP has made and it does not appear to be dynamic.--Jorfer (talk) 22:22, 3 January 2009 (UTC)
- I agree that it seems plausible that the three edits by 98.223.88.40 were made by someone connected closely with the article, perhaps Petergans. However, the Wikimedia software logs out users after an hour of inactivity, and it is easy to make an edit accidentally while not logged in, thinking that one is logged in. In particular, it is completely inappropriate to accuse an editor of sockpuppetry, when an honest mistake is just as likely an explanation. Further, this is an issue that is best raised on user talk, not article talk. In any case, assumption of good faith is the best way to de-escalate, rather than escalate a disagreement. Geometry guy 22:51, 3 January 2009 (UTC)
- Whether it was intentional or not was not the point. The point was Petergans' behavior with this article.--Jorfer (talk) 23:18, 3 January 2009 (UTC)
- The IP is an ISP based in New Jersey, whereas Petergans is based in Leeds, so even the presumption of the identity of the IP is inappropriate. Geometry guy 23:29, 3 January 2009 (UTC)
- Fair enough, I was hoping someone else could check since I am not an administrator. Just a random coincidental (since it occurred right after Petergans edit) IP interjection...weird but OK. That only leaves the strikethrough edit and argument on several attempts at opening access that have been denied.--Jorfer (talk) 05:18, 4 January 2009 (UTC)
- The IP is an ISP based in New Jersey, whereas Petergans is based in Leeds, so even the presumption of the identity of the IP is inappropriate. Geometry guy 23:29, 3 January 2009 (UTC)
- Whether it was intentional or not was not the point. The point was Petergans' behavior with this article.--Jorfer (talk) 23:18, 3 January 2009 (UTC)
- I agree that it seems plausible that the three edits by 98.223.88.40 were made by someone connected closely with the article, perhaps Petergans. However, the Wikimedia software logs out users after an hour of inactivity, and it is easy to make an edit accidentally while not logged in, thinking that one is logged in. In particular, it is completely inappropriate to accuse an editor of sockpuppetry, when an honest mistake is just as likely an explanation. Further, this is an issue that is best raised on user talk, not article talk. In any case, assumption of good faith is the best way to de-escalate, rather than escalate a disagreement. Geometry guy 22:51, 3 January 2009 (UTC)
- If it wasn't for this edit, I probably wouldn't be accusing him of such. Since IP edits are few and far between on this page, and since the IP is exhibiting extreme anger, it does not appear to be someone detached from the article. These are the first edits this IP has made and it does not appear to be dynamic.--Jorfer (talk) 22:22, 3 January 2009 (UTC)
- Whatever disagreements you may have with Petergans, as far as I have seen he is unfailingly polite and I doubt very much that that hostile IP is him. Regarding the edits themselves, in my opinion he is correct in removing the inaccuracies and misconceptions that you have introduced as a result of your limited understanding of the topic. I suppose that's the reason the IP removed them too, although with a less civil edit summary. --Itub (talk) 17:19, 3 January 2009 (UTC)
My problem with the most recent edits was the removal of this which serves to increase access:
The graphs on the right have been created based on the definition of Ka at the top of the page and each species pKa values to aid in explaining the mathematical implications of pKa on speciation (the formation of species).
--Jorfer (talk) 22:54, 3 January 2009 (UTC)
- This was removed because it is incorrect and confusing. Petergans (talk) 10:31, 4 January 2009 (UTC)
Solution equilibria
I have just seen that stability constants of complexes has been promoted for Did You Know and will appear on the front page in the next day or two. This article is complementary to ADC. It rounds off the project, which I began last May, to bring solution equilibria in WP up to a decent standard, from the deplorable state that it was in before. The main changes I have made are:
- chemical equilibrium - rewritten
- equilibrium constant - new
- determination of equilibrium constants - new
- stability constants of complexes - new
- acid dissociation constant - contributed
Regarding the last two comments in base edits above, if and when user:proteins is willing to revise the article I will be happy to cooperate with him. Until that time I'll keep watching ADC, but will only intervene in exceptional circumstances. Petergans (talk) 16:27, 7 January 2009 (UTC)
Intro Length
Jeeze. Theres just no pleasing everyone. (See copious argument about the lead during FAC). EagleFalconn (talk) 07:13, 20 January 2009 (UTC)
Standard free energy change definition
The article says...
- Note that the standard free energy change for the reaction is for the changes from the reactants in their standard states to the products in their standard states.
This is not consistent with my (somewhat limited) intuition. Am I incorrect in thinking that the standard free energy change for the reaction is for the change from all reactants and products in their standard states to the equilibrium mixture of reactants and products? Awaspaas (talk) 04:08, 8 September 2009 (UTC)
- Standard free energies are given as if the reaction goes to completion I believe. This is of course a theoretical free energy change as even reactions like the combination of Sodium and Chlorine leaves Schottky defects and Frenkel defects in the latice structure though they are considered to go to completion. This theoretical value is still useful as it can be adjusted for actual yield of the product and can be used for very close approximations where the reaction is considered to go to completion.--Jorfer (talk) 05:39, 8 September 2009 (UTC)
- Now that I think of it, those two circumstances may be referring to the same thing, but I'm not sure. If we have a spontaneous reaction of a reactant becoming a product where the product is favored, then the theoretical conversion from pure reactant to pure product would be accompanied by a decrease in free energy. Likewise if a mixture of reactant and product in their standard states were allowed to reach equilibrium, the reaction would proceed in the forward direction to produce more product and consume more reactant, again accompanied by a reduction in free energy. But are those two reductions in free energy the same value? Awaspaas (talk) 21:32, 10 September 2009 (UTC)
- You are right that any reaction will eventually go to completion given enough time, and thus will equal the value for Free Energy if standard conditions remain. The key word is given enough time; due to kinetics that can be a near infinite period of time. However, the values are given per mole of reacted material (rather than total material), so the values are to be adjusted for actual yield, making it useful not just in theory but in practice.--Jorfer (talk) 22:40, 10 September 2009 (UTC)
- If you are calculating a ΔrG from tabulated values of ΔfG, then you are calculating it for 100% completion. If you get a value which is very close to zero, you know that the reaction will be an equilibrium: on the other hand, if ΔrG is lower than about −60 kJ/mol (−15 kcal/mol) you pretty safe ignoring any equilibrium effects. The equibrium pressure of free chlorine above solid sodium chloride (ΔfG = −384 kJ/mol) is completely negligible! Table salt simply doesn't spontaneously revert to sodium and chlorine! Physchim62 (talk) 10:52, 11 September 2009 (UTC)
- You are right that any reaction will eventually go to completion given enough time, and thus will equal the value for Free Energy if standard conditions remain. The key word is given enough time; due to kinetics that can be a near infinite period of time. However, the values are given per mole of reacted material (rather than total material), so the values are to be adjusted for actual yield, making it useful not just in theory but in practice.--Jorfer (talk) 22:40, 10 September 2009 (UTC)
The numerical value for the standard Gibbs energy change is based on the complete conversion of one equation mole from the left side to the right side of the equation. One equation mole means that if the equation contains a stoichiometry coefficient other than one for either a reactant or product, that number of moles of that reactant must be converted or product formed. For reactions in solution, if the reaction is reversible, the concentration of reactants and products must remain at standard state throughout the reaction process. There are various hypothetical ways of achieving this result. 1) You have an Atlantic ocean of reactant and product, so that the conversion of 1 mole does not significantly change concentrations. or 2) You constantly replenish reactant and remove excess product to maintain everything at standard state, or 3) you allow the reaction to proceed converting an infinitesimally small amount of reactant to product such that the concentration change on either side of the equation is negligible, and factor the measured result to obtain kJ/mol. For biochemical reactions, ΔG°' is typically determined by measuring equilibrium constants and using -ΔG°' = RT ln K. 96.54.32.44 (talk) 06:01, 27 February 2011 (UTC)
Ionic Strength
Is there a particular reason why the effect of ionic strength on the the pKa is not dealt with directly? And presentation of the "Debye-Huckel" relationship that can be used to provide the apparent pKa based on the pKa and ionic strength? --Dr DBW (talk) 23:50, 8 October 2009 (UTC)
It is not encyclopedic to demonstrate all forms of a mathematical equation. Wikipedia is not a textbook.--Jorfer (talk) 22:13, 10 October 2009 (UTC)- It is not a simple matter. I am currently working on this: see Bromley equation, Davies equation and SIT theory which I have written. It remain to write Pitzer equation but I don't know when I will have the time to do it. Petergans (talk) 08:09, 12 October 2009 (UTC)
- Good stuff :thumbsup: Just wanted to see if there was something I was missing to why it wasn't in there, and seems rather fundamental (to me anyone, but hey, that is where my interst lays) --Dr DBW (talk) 00:18, 14 October 2009 (UTC)
- It is not a simple matter. I am currently working on this: see Bromley equation, Davies equation and SIT theory which I have written. It remain to write Pitzer equation but I don't know when I will have the time to do it. Petergans (talk) 08:09, 12 October 2009 (UTC)
standards states in mixed solvents
I noticed that in the section discussing mixed solvents (water/meoh for example) for those species which are not normally soluble in water, the it was stated that there is currently no method to mix standard states. This does not reflect the current literature, as the Born Radius model has been used to adjust the standard states for at least 20-years. See for example literature by Chen and Mock. 67.136.192.10 (talk) 14:37, 12 October 2009 (UTC)elephantwalker
polyprotic acid: fractional formation
Unregistered user:Quantumkinetics added the following explanation for the speciation diagram.
The plots on the right were generated by calculating the percent formation of each species in solution. These values are also known as fractional composition values, α (alpha). In general, the fractional composition of a n -protic acid that has been deprotonated i -times is given as a function of pH (or [H+]) by the equation:
where K0 = 1 and the other K-terms are the dissociation constants for the acid. So for a generic diprotic acid, 3 species are present in solution: H2A, HA-, and A2-. The fractional composition values can be calculated as shown:
It is more complicated than it needs to be because a product such as is simply the cumulative constant βj and the denominator is, of course, the same in all expressions of fractional formation . Moreover, the equations do not apply at high pH where the self-dissociation of water must also be taken into account.
Since the specition diagrams were not calculated in this way, the expressions have been removed to this discussion to avoid giving a misleading impression. Petergans (talk) 08:14, 13 June 2010 (UTC)
More on unfavorable entropy contribution
- First a note to Petergans: Yes, Levoslashx's edit today is incorrect. But I think a detailed edit summary (35 words) deserves more than a one-word dismissal as "ignorance". This is a good-faith edit, which a confused student could make. Perhaps an explanation of the error can help clarify the article for others.
- The source of his/her error seems to be that the table has a column heading (-)T ΔS, which does have positive values. I will change the signs to list (+)T ΔS with negative values.
- The edit summary asks "If the enthalpy is positive, and the entropy contribution was unfavorable, how could it be spontaneous?" The answer is that these are standard-state values (indicated by the superscript
 , valid when reactants and products are all at unit activity (1 mol/L for ideal solution). This is noted in the last paragraph of the Thermodynamics section. In the standard state it is true that the ionizations are not spontaneous. However they do have small non-zero equilibrium constants, meaning that they are spontaneous when the ion concentrations are sufficiently small (Q < K).
, valid when reactants and products are all at unit activity (1 mol/L for ideal solution). This is noted in the last paragraph of the Thermodynamics section. In the standard state it is true that the ionizations are not spontaneous. However they do have small non-zero equilibrium constants, meaning that they are spontaneous when the ion concentrations are sufficiently small (Q < K). - Finally the physical reason for the negative entropy of ionization is that the ions formed tend to organize the solvent water molecules around them, which decreases the
ionizationentropy of the system. Dirac66 (talk) 16:40, 26 February 2011 (UTC)
- I posted a detailed explanation on User talk:Levoslashx at the time of the reversion.
- I was following the widely used convention as in the cited references.
- Of course there is spontaneous dissociation when HA is added to water. The significance of the equilibrium constant is that it allows the extent of dissociation to be calculated, as in ice table. What does "However they do have small non-zero equilibrium constants" mean? Equilibrium constants are, by definition, independent of concentration, apart from some possible variation due to changes in the quotient of activity coefficients.
- I am aware of the explanation, but could not find a citation to verify it. Petergans (talk) 17:32, 26 February 2011 (UTC)
- OK, good.
- Yes, I know many authors list (-)TΔS as it is easier to put only one minus sign in the table. But I think this way is clearer as it is harder for a reader to miss a whole column of minus signs than to miss one as Levoslashx seems to have.
- Here I did not mean to suggest that K depends on concentrations, but rather that the spontaneity (sign of ΔG) depends on concentrations through ΔG = ΔGo + RT ln Q. My answer #3 above is too long for this article, but after the words "dissociation of a weak acid is not a spontaneous process" we could add "when reactants and products are in their standard states", or just "in the standard state".
- Atkins' Physical Chemistry (8th edn p.94) mentions that ion entropies vary in relation to their degree of ordering the surrounding water. I will try to write a sentence or two based on this. Dirac66 (talk) 23:14, 26 February 2011 (UTC)
I have added a ΔG column to the table, and reverted to tabulating minus T ΔS. It should be much clearer now that ΔG = ΔH - TΔS. Petergans (talk) 16:12, 28 February 2011 (UTC)
Measurement of pKa of strong(ish) acids
I'm having trouble with this paragraph, which may have become garbled by contributions from different editors:
"In water, measurable pKa values range from about −2 for a strong acid to about 12 for a very weak acid (or strong base). All acids with a pKa value of less than −2 are more than 99% dissociated at pH 0 (1 M acid). This is known as solvent leveling since all such acids are brought to the same level of being strong acids, regardless of their pKa values. Likewise, all bases with a pKa value larger than the upper limit are more than 99% protonated at all attainable pH values and are classified as strong bases.[3]"
"measurable pKa values range from about −2 for a strong acid" I would have thought that the measurable range in water would be +2 to 12[**]. At low pH we have the problem of what is meant by pH itself, plus the buffering due to H2O accepting protons from the strong acid, although there are equations[*] that adjust for water ionization. Likewise at very high pH, H20 buffers by loss of protons.
"All acids with a pKa value of less than −2 are more than 99% dissociated at pH 0 (1 M acid). This is known as solvent leveling since all such acids are brought to the same level of being strong acids, regardless of their pKa values." These two sentences seem either to be non sequitur. or don't explain the connection completely.
Can I suggest the following rewrite?
In water, readily measurable pKa values range from just below 2 for a strongish acid to slightly greater than 12 for a very weak acid (conjugate of a strongish base). For stronger acids, H2O itself acts as a buffer, accepting H+ from the strong acid, and limiting the change in pH. Differences in definition of pH in terms of activity versus concentration also make measurement difficult when [H+] is high. As a result, acids with very negative pKa do not lower the pH below zero in aqueous solution even though an acid with pKa -2 should be 99% dissociated at pH 0. This is known as solvent leveling since all such acids are brought to the same level of being strong acids, regardless of their pKa values. Very low pKas can be determined using polar solvents other than water. A similar buffering effect due to donation of H+ from H2O occurs at pH 14, limiting the measurable pKa of very weak acids (conjugate acids of strong bases).
[*] I encountered this equation many many years ago when I taught phys chem for biochemists. As I recall, the textbook was Chang which I no longer have in my possession.
[**] I think the workable range is more like 1.5 - 12.5 by maybe we don't want to get into fractional values. 96.54.32.44 (talk) 19:44, 26 February 2011 (UTC)
- This does seem clearer. Two suggestions for alternate wording:
- "strongish" is not an English word. From memory, the usual term is "fairly strong".
- "buffer". I don't recall seeing "buffer" used in this context, although I suppose the effect is analogous to buffering. Usually one says that H3O+ is the strongest acid that can exist in water, since any stronger acid transfers a proton to H2O and forms H3O+. And similarly OH- is the strongest base that can exist in water. Dirac66 (talk) 23:33, 26 February 2011 (UTC)
- It is indeed buffer action in that H2O is mopping up H+ from the strong acid, and limiting further change in pH. It's unfamiliar in that we don't usually think of buffers having pKa 0 or pKa 14, nor do we normally expect buffers to be present at 55 mol L-1. The relevant buffer equations are
- pKa = 0
- H3O+ ⇌ H2O + H+
- H+ here represents protons from the strong acid; addition of strong acid drives the reaction right to left.
- pKa = 14
- H2O ⇌ OH- + H+
- H+ here represents protons captured by the strong base; addition of strong base drives the reaction left to right. 96.54.32.44 (talk) 02:09, 27 February 2011 (UTC)
- The usual definition of a buffer solution is a conjugate acid-base couple. So to be consistent we should say that the buffer at pH 0 is the couple H2O - H3O+, rather than only H2O. And similarly the buffer at pH 14 is the couple H2O - OH-. Dirac66 (talk) 02:45, 27 February 2011 (UTC)
- Yes, that's it exactly. 96.54.32.44 (talk) 06:04, 27 February 2011 (UTC)
- The usual definition of a buffer solution is a conjugate acid-base couple. So to be consistent we should say that the buffer at pH 0 is the couple H2O - H3O+, rather than only H2O. And similarly the buffer at pH 14 is the couple H2O - OH-. Dirac66 (talk) 02:45, 27 February 2011 (UTC)

It is true that the buffer capacity of water increases steeply at high hydrogen ion concentrations, see diagram at right. (I will add this and the equation on which it is based to buffer solution). Nevertheless, there is a fundamental flaw in the arguments above, which is, that all pK determinations are based on pH measurements. At very high or low hydrogen ion concentrations pK values are obtained as concentration quotients from spectrophotometric measurements. The hydrogen ion concentration is calculated from its experimentally known analytical concentration. There is always the possibility of confusion when the word pH is used. The glass electrode can be used to measure H+ activity or concentration depending on how it is calibrated (buffer solutions or acid/base titration). When calibrated in concentration the measurement should be written as p[H] but this convention is not universally accepted.
The lower limit of -2 is a theoretical limit which is based on the calculated extent of dissociation of an acid in 1M H+ (concentration) solution. It is stated to be approximate. BTW I deprecate the use of weasel words such as "readily", "strongish" in this context, as pK is a quantitative measure, not a qualitative one. The term "strong acid" comes to us from the past, as an acid which is "fully dissociated". Quantitatively there is no such thing as 100% dissociation, so we have to interpret "fully" as meaning that the undissociated acid concentration is below the limit of measurability. I will add the missing link to solvent leveling which should help to explain that sentence. Petergans (talk) 10:38, 27 February 2011 (UTC)
- Um, why does your graph have a local maximum at pH 7? I learned that pure water has a negligible buffer capacity, since there is no conjugate acid-base couple which is effective at pH 7. Dirac66 (talk) 14:16, 27 February 2011 (UTC)
- What's being shown is the buffering capacity as represented by the lowest concentration of a member of a buffering couple. At pH 7, this is 10-7 mol/L. At pH 6 [H+] is 10-6 mol/L but [OH-] is 10-8 mol/L. Hence buffering capacity seems to go down. At low pH, the H2O / H3O+ couple starts to dominate, so buffering capacity starts to rise again. Same happens at high pH when the OH- / H2O couple dominates. The objection might be that OH- / H+ are not members of a buffer couple, so why don't we treat H2O / H3O+ as the operative buffer couple at pH 6? 96.54.32.44 (talk) 22:33, 27 February 2011 (UTC)
- Unless what is depicted is the buffer capacity of a pH 7 buffer solution rather than pure H2O. 96.54.32.44 (talk) 23:38, 27 February 2011 (UTC)
Just so. See buffer solution for the updated explanation. Petergans (talk) 13:57, 28 February 2011 (UTC)
For HI entropy favors dissociation
From W.E.Dasent "Inorganic Energetics" (2d edn 1982, Cambridge Univ Press) p.168:
- Reaction HX(aq) → H+(aq) + X-(aq) X = F Cl Br I
- ΔH° / kJ -16 -57 -65 -62
- (-)TΔS° / kJ (*) +30 +10 +4 -3
- ΔG° / kJ +14 -47 -61 -65
- Larger monoatomic anions are less effective in ordering solvent, and this trend contributes to strengthening the acid. For HI Ka is estimated at 1011. I will think about how to put this into the article.
- (*) I have put a minus before the entropy term for consistency with the article. However Dasent is one source who gives the values of (+)TΔS°. Dirac66 (talk) 01:04, 1 March 2011 (UTC)
- My last attempted post seems to have disappeared, in which I explained the addition of a ΔG column and reversion of sign for TΔS. The idea is to show the components of ΔG that are added together. Showing ΔG explicitly makes this clear.
- The Dasent example is dubious since K has not been measured except for HF. In any case, I did not include any comment on the fact that ΔS is negative because it would have been based on speculation. Though it is well-founded it's not the same as experimental evidence. The problem is that simulation of ionic solutions is extremely complicated. M.R. Wright, "An introduction to aqueous electrolyte solutions, Wiley, 2007 has a good discussion of computer simulations for studying solvation, chapter 13, pp 542-560, but I think it's a bit off-topic for this article. Petergans (talk) 09:34, 1 March 2011 (UTC)
- Sorry - I confused you by moving your last post to the end of the second previous section because it was part of that discussion. I did note the move in my edit summary.
- It is true that Dasent's values for HCl, HBr and HI are only theoretical estimates, so the conclusion that TΔS is positive (or (-)TΔS is negative if you prefer) is not very definitive. But we can use such data for HCl, HBr, HI to say that the entropy contribution is less important for anions of large atoms, and that it has even been estimated to be of opposite sign in the case of HI. Dirac66 (talk) 14:28, 1 March 2011 (UTC)
- The Dasent example is dubious since K has not been measured except for HF. In any case, I did not include any comment on the fact that ΔS is negative because it would have been based on speculation. Though it is well-founded it's not the same as experimental evidence. The problem is that simulation of ionic solutions is extremely complicated. M.R. Wright, "An introduction to aqueous electrolyte solutions, Wiley, 2007 has a good discussion of computer simulations for studying solvation, chapter 13, pp 542-560, but I think it's a bit off-topic for this article. Petergans (talk) 09:34, 1 March 2011 (UTC)
I have removed the reference to spontaneous, because it was wrong to include it (my mistake!). A reaction is spontaneous if ΔG is negative, not ΔG![]() . The discussion of entropy looks just about right as it is. I have not been able to find anything more specific in the books available to me here. Petergans (talk) 07:50, 2 March 2011 (UTC)
. The discussion of entropy looks just about right as it is. I have not been able to find anything more specific in the books available to me here. Petergans (talk) 07:50, 2 March 2011 (UTC)
On borate ionization, hydrolysis issue
It may be that this citn. will be good enough, but something deeper would be nice:
A. Earnshaw & Norman Greenwood, 1997. Chemistry of the Elements, 2nd Edn. Butterworth-Heinemann, pp. 203-205. [ ISBN13 978-0750633659 ]
Prof D. —Preceding unsigned comment added by Meduban (talk • contribs) 17:37, 13 May 2011 (UTC)
- The boric acid article refers this fact to W.L. Jolly, Modern Inorganic Chemistry, McGraw-Hill 1984 (p.198), which I have just rechecked. The conclusion is based on the Raman spectrum of B(OH)4- observed in strongly alkaline solutions. Yes, the source should be given in this article also, so I'll copy it in. I don't have a copy of Earnshaw + Greenwood to check, but please add it too if it provides additional support for the statement. Dirac66 (talk) 19:30, 13 May 2011 (UTC)
"(B(OH)3) acts as a weak acid, and is sometimes described as a proton donor ". This is incorrect - candidate for my howler list?. It is described as behaving like a proton donor. Petergans (talk) 08:07, 14 May 2011 (UTC)
- "Behaving like a proton donor" is more correct but possibly unclear to nonexpert readers. How about "behaving like a proton donor in increasing the concentration of H3O+"?
- Also I have found two more sources for the formation of B(OH)4-. Cotton and Wilkinson "Advanced Inorganic Chemistry" 5th edn p.169, and Housecroft and Sharpe "Inorganic Chemistry" 2nd edn p.315. Jolly is still the best source however because it indicates that the conclusion is based on the Raman spectrum. Dirac66 (talk) 17:16, 14 May 2011 (UTC)
- This is a very old story, predating C&W and H&S by at least half a century, i.e. my student days -:(. I don't know where the original proposal came from, but I guess it was based on thermochemical evidence about bond energies and the calculated OH bond dissociation energy. Petergans (talk) 09:29, 15 May 2011 (UTC)
- OK then, I have inserted "confirmed by Raman spectroscopy" here and at boric acid. This removes the suggestion that Raman was the first evidence, while still citing Jolly as a reliable source. Dirac66 (talk) 14:08, 15 May 2011 (UTC)
- This is a very old story, predating C&W and H&S by at least half a century, i.e. my student days -:(. I don't know where the original proposal came from, but I guess it was based on thermochemical evidence about bond energies and the calculated OH bond dissociation energy. Petergans (talk) 09:29, 15 May 2011 (UTC)
pH (Help for the Layman)
Please let me know if there is a more appropriate forum for my issue: I'm not suggesting dumbing this article down, just adding something to make it useful to the layman. I have tried to understand this article a couple times, but I don't see any way to relate it to pH. The average education includes an introduction to the pH scale, and most people can understand the comparative acidity or alkalinity of a substance by its location on that scale. Since the infobox on such articles as Citric Acid provides pKa values rather than pH, and the Acidity link comes to this page, I think it behooves us to at least offer a way for the layman to convert pKa into pH, assuming that's even possible. If not, then I guess I'll see if there's a way to have the pH available in addition to the pKa.
Your average Joe just wants to know, "Is citric acid more acid than acetic acid?", or something like that. Wikipedia should be able to provide that without sacrificing the technical creamy-center goodness. Thank you for your forbearance. Keyesc (talk) 02:11, 8 December 2011 (UTC)
- Briefly, the pH of an acid solution depends on both the pKa of the acid AND its concentration. So the comparison would have to be done at equal concentrations for example. We could however add a section explaining how the pH is calculated for an example or two. Dirac66 (talk) 03:49, 8 December 2011 (UTC)
The smaller the pKa, the more acidic the compound. pH is dependent on concentration, so it is pretty much meaningless on its own. The concentration of H+ ions for a 1 M solution of a weak acid (HA) in water can be calculated:
Making the assumption that all the H+ ions are derived from the acid, HA, we can approximate [H+] = [A<sup.>-]. Rearranging, we can derive an expression for the concentration of H+ ions, [H+]
pH is simply -log [H+]
Do note that many assumptions are used here. The bottom line is that pH and pKa is not trivially convertible. pH defines the acidity of a specific solution, whereas pKa is a measure of how ionizable any hydrogen substituent is (one molecule has one pKa for each hydrogen). --Rifleman 82 (talk) 03:56, 8 December 2011 (UTC)
- Okay, thanks for this. We definitely need to include a section on pH prominently here, since this is the only measure of acidity your average reader will ever have met. Maybe a second or fourth sentence should be added along the lines of 'Unlike pH, which is a measure of the acidity of a particular concentration of an acid, pKa measures the acidity of a chemical directly - so hydrofluoric acid always has a pKa of 3.17, but if it is diluted then the pH of the solution will depend on how much water it is mixed with.' --87.242.195.105 (talk) 18:43, 17 June 2014 (UTC)
Actually, the concept of pH has a much wider application than simple acids. It's not something that can be simplified down to a sentence or two. For example, the pH of a solution of a base can be calculated from pKb which is related to pKa ... Also, salts like Al2(SO4)3 and FeCl3 dissolve in water to give acidic solutions. I think that what the layman needs is a qualitative exposition of the idea of acidity, but this is not the place for that. This article is specifically about acid dissociation constants. By their very nature they are quantitative, not qualitative. The article on pH is more directed towards the layman.Petergans (talk) 22:01, 20 June 2014 (UTC)
pH/pKa inconsistency in phosphoric acid
Just as a small comment: in acid dissociation, when the acid and conjugate base are equal in concentration, then pH = pKa. for the graph depicting phosphoric acid dissociation, this is incorrect; the first pKa equals 2.15, and in the graph, the 50/50 distribution quantities is not at that value. any suggestions? 95.172.88.5 (talk) 14:16, 25 September 2014 (UTC)
- Yes, you are correct. The graph appears to show pKa1 at about 3.1, so it needs to be redrawn. Dirac66 (talk) 03:12, 26 September 2014 (UTC)
- I agree. It looks as though I used a different value of pK1 in the table and when creating the graph.I'll have to check which is the "correct" value to use. I will attend to this in the next few days. Petergans (talk) 10:00, 26 September 2014 (UTC)
So...
Perhaps this article should be completely re-written as it is terribly unencyclopedic and requires pre-existing specialist or expert knowledge of chemistry to comprehend and is of little if any educational value to the casual reader. Compare to the "internal combustion" article, while it describes a complex system of many interacting physical and chemical processes, it is concise and accessible to the intended audience and 25% shorter than this article. Just as it would be inappropriate for a chemist or chemistry student to rely on a general knowledge encyclopedia as a primary source for information on a chemistry topic, the reverse is true, as a lay person seeking basic information on a topic consults an encyclopedia rather than a detailed technical textbook . That is where this article belongs in a text book or a professional reference.
107.31.174.187 (talk) 06:01, 8 December 2011 (UTC)Moi
I think you are mistaken in thinking that pH is a property specific to a compound, because it isn't. pKa is the correct measure of the acidity of a hydrogen substituent of a compound, and that's exactly what is said in the first sentence of the article. This topic is pretty basic, and it might be covered at the upper secondary school level, or in the first year of undergraduate chemistry. This topic is perhaps the foundation for a topic like the Henderson-Hasselbach equation, for example. There is a little math but that is quite unavoidable. --Rifleman 82 (talk) 06:16, 8 December 2011 (UTC)
Actually, yes I was under that incorrect pH assumption, perhaps the lead could explain the concept in a more simplified rudimentary style and include an explanation on the differences of the concept of pH and pKa. The extent of my formal science education was an introductory Physics 101 class so I was until now ignorant of the acidity constant until reaching this article. P.S. Thank you for proving wrong the stereotype commonly held by many people who regard infantrymen as having have a rather limited intellectual capacity. :) 107.31.174.187 (talk) 07:09, 8 December 2011 (UTC)Moi
- The fundamental difference between the IC engine a pKa value is that an acid dissociation constant is a quantitative measure of a molecular property, so a qualitative presentation cannot convey the essence of the concept.
- Secondly, there is an inherent contradiction between making something of this nature comprehensible to the layman and using proper scientific rigour. I opt for scientific rigour. In my view, the alternative is waffle and Wikipedia has far too much of that already.Petergans (talk) 11:39, 8 December 2011 (UTC)
Calculation of the Ka value
For example:
Ka(HCl) = [Cl−
]×[H+
]÷[HCl] = (Density(Cl−
)÷Molar mass(Cl−
))×(Density(H+
)÷Molar mass(H+
))÷(Density(HCl)÷Molar mass(HCl)) ≈ (?g/L÷35.45g/mol)×(?g/L÷1.01g/mol)÷(1.49g/L÷36.46g/mol) ≈ ?mol/L×?mol/L÷0.04mol/L
But how to get the Density(Cl−
) and Density(H+
)? Or is it possible to get the [Cl−
] and [H+
] directly?
Thanks. 123.119.16.126 (talk) 13:01, 27 May 2014 (UTC)
- No, it is not possible to measure the density of each component of a solution. The classical method determines the ion concentrations by measuring the electrical conductivity of the solution, knowing the molar conductivity. For HCl, the ion concentration is about equal to the acid concentration, which shows that the acid is 100% dissociated and is a strong acid. For a weak acid such as CH3COOH, the conductivity gives the ion concentrations which allow Ka to be calculated as in your first line. Other methods can also be used to measure the concentrations, such as spectrophotometry. Dirac66 (talk) 19:12, 27 May 2014 (UTC)
- Perhaps a link between molar conductivity and activity coefficient is required for strong electrolytes and the hypotthesis of full dissociation is not tenable.--188.26.22.131 (talk) 15:59, 26 September 2014 (UTC)
Standard state symbol
I switched from a graphics-file symbol to a unicode character that looked about the same, in order to give better display when the font-size or background-color is not what is hardcoded into the graphics file. I chose the character based on the character-equivalents noted in the file itself by User:Pieter Kuiper and in keeping with what Standard state says IUPAC recommends. I called it a plimsoll since that was an easy thing to say and link in my edit-summary, but that is maybe an older or typographic term not commonly used in chem and/or wouldn't technically have the same bar length as the chemistry symbol. But call it what you like..."ominus" ( seems to be used in chem/physics style-guides for LaTeX), "strikethrough-O" (File:StrikeO.png is file I swapped out) or U+29B5 ⦵ CIRCLE WITH HORIZONTAL BAR) (that's the unicode char I swapped in)...that it's a valid symbol here is supported by WP:RS. @Petergans:, what are the specific differences in the symbol you are seeing and what is the basis for choosing? DMacks (talk) 15:06, 30 April 2015 (UTC)
- Unfortunately the symbol inserted by DMacks appears not to be supported by all browsers. Both my Firefox and my Explorer show it as a blank square (not circle), suggesting that the browsers do not recognize the symbol. From his comments I think DMacks sees it (presumably in a different browser) as a barred circle of slightly more appropriate proportions than the symbol used by Petergans. This may be true in some browsers, but in the interests of having a symbol which everyone can see, I am going to revert to Petergans' version of a plimsoll. (Such problems did not arise with paper encyclopedias :-)) Dirac66 (talk) 15:20, 30 April 2015 (UTC)
- How does
olook for you? DMacks (talk) 15:40, 30 April 2015 (UTC)- Yes, that is indeed visible in both Firefox and Explorer, presumably because it is just Wikipedia source code without the special Unicode character. Dirac66 (talk) 16:22, 30 April 2015 (UTC)
- I also saw the blank square on my browser. The symbol
ois too small.0orOmay be OK for text, but neither corresponds exactly to the symbol used in books. I considered them, but decided in the end on the graphic. Incidentally, this symbol may appear in many articles where thermodynamic properties are discussed. The representation in equations,e.g. , is not wholly satisfactory either. Petergans (talk) 18:51, 30 April 2015 (UTC)
- I also saw the blank square on my browser. The symbol
- Yes, that is indeed visible in both Firefox and Explorer, presumably because it is just Wikipedia source code without the special Unicode character. Dirac66 (talk) 16:22, 30 April 2015 (UTC)
- How does
This was settled last year, but yesterday editor 86.145.72.84 seems to have reinserted the symbols which are illegible in Firefox and Explorer into the Thermodynamics section. In the interests of having a Wikipedia article which is legible in all browsers, I will restore the ![]() symbols, without reverting his/her edits entirely as they do contain other useful changes. Dirac66 (talk) 23:47, 2 April 2016 (UTC)
symbols, without reverting his/her edits entirely as they do contain other useful changes. Dirac66 (talk) 23:47, 2 April 2016 (UTC)
Kw
In the section on self-ionization of water the reaction is given as
- H2O ⇌ OH− + H+
Why H+ and not H3O+? The answer is that the aquation of the proton is not an equilibrium process - it goes to 100% completion. It is therefore irrelevant to Kw, which is an equilibrium constant. When the proton is involved in chemical equilibria in water it is a universal convention to represent it as H+, even though the species in solution is the hydrated hydronium ion. Petergans (talk) 08:59, 7 August 2015 (UTC)
- H3O+ is a more correct (though not exact) description of the cation in water than is H+, so the equation 2 H2O ⇌ OH− + H3O+ is really more correct. It is true that a common convention is to simplify the equation as you have done, both because it is simpler and for historical reasons: Arrhenius believed the cation was H+ before Bronsted modified it. But there is not a universal convention to always write it that way. Of the two general chemistry books on my desk, Whitten et al (4th ed. p.359) first writes the H3O+ equation and then says In simplified notation and gives the H+ equation, while Petrucci et al. (8th ed. p.672) and only gives the H3O+ equation.
- In summary both versions are valid and widely used. So I think that to be of maximum help to uninitiated readers, this section should include both versions and make clear that chemists treat them as equivalent. Dirac66 (talk) 21:29, 7 August 2015 (UTC)
- I fear that we would be perpetuating a text-book error. Kw refers to an equilibrium, so its value is independent of any non-equilibrium process. Thermodynamically, nothing is gained by putting another water molecule on both sides of the equilibrium expression. In reality the hydronium ion is further aquated. Why not go all the way and use H9O4+? I prefer the common convention that, in solution, H+ refers the the solvated hydrogen ion. This convention applies to other aquo-ions like K+ whose hydration structure in aqueous solution is not known in detail.Petergans (talk) 07:36, 8 August 2015 (UTC)
- Wikipedia depends on sources, so before deciding that textbooks are in error we need to find other sources. I have now checked Housecroft and Sharpe's Inorganic Chemistry (2nd ed p.163) which also writes only the equation with H3O+, but does add the explanation that In aqueous solution, protons are solvated and so it is more correct to write H3O+(aq) than H+(aq). Even this is oversimplified because the oxonium ion is further hydrated and species such as H5O2+, H7O3+ and H9O4+ are also present. So we can use this as a source (rather than general chemistry books) and refer to H3O+ as well as the larger cations. I do not object to also mentioning that H+(aq) is used as a common abbreviation for the whole set, but I do object to completely suppressing mention of H3O+ in this section. Dirac66 (talk) 16:30, 8 August 2015 (UTC)
- I also consider the point about H+ + H2O → H3O+ being a nonequilibrium to be irrelevant because all modern chemists understand that H+(aq) not a bare proton but an abbreviation for H3O+(+ H5O2+ + ...). So what we are calling H+ is actually the same as H3O+ (except for the added H5O2+ etc.) and there is no conversion to consider. The real question is which formula is most helpful to the reader: H3O+ is closer to the truth than H+ taken literally (as a bare proton), but H+ is used by chemists as an abbreviation for the complete mixture of all the hydrated cations so it can be considered exact provided the reader understands it. That is why it is essential to explain the meaning of H+(aq) at least. Also since many authors do use H3O+ as an approximate description, it is important to mention it as well although yes, we can explain that it is incomplete. Dirac66 (talk) 17:31, 8 August 2015 (UTC)
- I fear that we would be perpetuating a text-book error. Kw refers to an equilibrium, so its value is independent of any non-equilibrium process. Thermodynamically, nothing is gained by putting another water molecule on both sides of the equilibrium expression. In reality the hydronium ion is further aquated. Why not go all the way and use H9O4+? I prefer the common convention that, in solution, H+ refers the the solvated hydrogen ion. This convention applies to other aquo-ions like K+ whose hydration structure in aqueous solution is not known in detail.Petergans (talk) 07:36, 8 August 2015 (UTC)
- We are here dealing with the conflict between thermodynamic concepts and molecular structure - neither has much to say to the other. Kw is a thermodynamic quantity and its value embraces the state of solvation of the hydrogen and hydroxide ions. I wish we could keep thermodynamics and molecular structure separate from each other, but it is a fact than they are scrambled together in many sources. In WP it behoves us to maintain the distinction, citing separate sources for the separate aspects. BTW, the same issue arises with metal complexes as in a reaction such as M+L=ML - the state of aquation of the metal ion is ignored when talking about the stability constant. Petergans (talk) 16:39, 9 August 2015 (UTC)
Reaction equilibrium formula error
In reference to Acid-base reaction#Acid-base equilibrium: In the following text I believe the Ka1 and Ka2 constants are on the wrong side of the first two equations, leading to another error where their ratio is reciprocated in the last equation.
The equilibrium constant for this reaction can be derived from the acid dissociation constants of adenine and the hydrogen phosphate ion.
[AH] = Ka1[A−][H+]
[H2PO4−] = Ka2[HPO42−][H+]
The notation [x] signifies "concentration of x". When these two equations are combined by eliminating the hydrogen ion concentration, an expression for the equilibrium constant, K. is obtained.
[AH][HPO42−] = K[A−][H2PO4−], K=Ka1/Ka2
The above formula contradicts the formula contained on Acid dissociation constant ...not being an expert I did not edit the entry. — Preceding unsigned comment added by DGElder (talk • contribs) 20:21, 22 January 2016
- The above was posted at WT:Be bold. I moved it to here, and formatted, and will ask for help at WT:WikiProject Chemistry#Acid-base reaction. Johnuniq (talk) 21:52, 22 January 2016 (UTC)
- There is no error. The expression
- [AH] = Ka[A−][H+]
- is a definition of Ka Petergans (talk) 11:52, 23 January 2016 (UTC)
- Um, yes, there is an error in this definition. The usual form of the definition is Ka = [A−][H+] / [AH]. If we isolate [AH], we have [AH] = [A−][H+] / Ka. Or equivalently Ka[AH] = [A−][H+]. The last form is what DGElder seems to have had in mind in saying that the Ka are on the wrong side.
- However the error is not in this article, but in Acid-base reaction#Acid-base equilibrium. I will check that article and make the necessary changes. Dirac66 (talk) 01:53, 24 January 2016 (UTC)
I hope you are right...
There is potential confusion relating to association and dissociation constants. My suggestion is always to write the definitions explicitly. For a monobasic acid
- [HA] = K[H+][A-], (dissociatoon)
- [H+][A-] = K[HA], , (association)
There is neither right nor wrong with these definitions. Both are equally valid, so it is important always to state explicitly which definition is being used. In the case of an article entitled dissociation constants the statement is in the title. Please note: the dissociation definition is preferred by organic chemists, but the association definition is used in general-purpose computer programs for equilibrium constant determinations (and hence publications) This is because association constants have always been used for metal complexes. For consistency stability constants are defined as association constants for both metal complexes and the ligands. Note also that pK for dissociation of a monobasic acid is numerically equal to the logarithm of the association constant.Petergans (talk) 10:32, 24 January 2016 (UTC)
- I agree that both dissociation and association constants are used in chemistry, so that it is necessary to make clear which is meant in each context. The discussion above refers specifically to Acid-base reaction#Acid-base equilibrium, where the text refers to acid dissociation constants of adenine and phosphate ions. No metal complexes are involved.
- In other contexts of course, association constants may be more appropriate. But not here. Dirac66 (talk) 16:35, 24 January 2016 (UTC)
picture

I have removed this pretty picture because it shows neither the pH values nor which indicator is responsible for the colour. If I knew which indicators were used I would be able to insert it in the appropriate article, which is not this one. Petergans (talk) 09:08, 27 January 2016 (UTC)
External links modified
Hello fellow Wikipedians,
I have just modified 2 external links on Acid dissociation constant. Please take a moment to review my edit. If you have any questions, or need the bot to ignore the links, or the page altogether, please visit this simple FaQ for additional information. I made the following changes:
- Added
{{dead link}}tag to http://sparc.chem.uga.edu - Added archive https://web.archive.org/web/20070205181504/http://jesuitnola.org:80/upload/clark/Refs/aqueous.htm to http://www.jesuitnola.org/upload/clark/Refs/aqueous.htm
- Added archive https://web.archive.org/web/20090331121853/http://chemaxon.com:80/marvin/sketch/index.jsp? to http://www.chemaxon.com/marvin/sketch/index.jsp
When you have finished reviewing my changes, please set the checked parameter below to true or failed to let others know (documentation at {{Sourcecheck}}).
This message was posted before February 2018. After February 2018, "External links modified" talk page sections are no longer generated or monitored by InternetArchiveBot. No special action is required regarding these talk page notices, other than regular verification using the archive tool instructions below. Editors have permission to delete these "External links modified" talk page sections if they want to de-clutter talk pages, but see the RfC before doing mass systematic removals. This message is updated dynamically through the template {{source check}} (last update: 5 June 2024).
- If you have discovered URLs which were erroneously considered dead by the bot, you can report them with this tool.
- If you found an error with any archives or the URLs themselves, you can fix them with this tool.
Cheers.—InternetArchiveBot (Report bug) 12:03, 3 October 2016 (UTC)
Basicity and acidity constants in infoboxes for metal hydroxides ?
The Wikipedia articles on several metal hydroxides have infoboxes with pKa and pKb values and no explanation. For example the infobox on calcium hydroxide says Acidity pKa 12.4 and Basicity pKb 2.37. That infobox has links for acidity and basicity which point to this article, which defines both Ka and Kb with reference to the usual Bronsted deprotonation and protonation reactions. However examples in chemistry books are usually organic, e.g. carboxylic acids and their anions, or amines and their cations. As a chemist I have difficulty believing that the values for Ca(OH)2 really refer to the formation of Ca(O)OH- and Ca(OH2)(OH)+ (??). I have found one website [here] which gives a similar value (2.43) for pKb with the explanation that it is for the Arrhenius-type dissociation of one OH-, in this case to give Ca(OH)+.
So we seem to have infobox values in other Wikipedia articles with a link to an explanation in this article which does not apply. What to do? Is this alternative Arrhenius-type definition of Kb for bases such as hydroxides widely accepted? (I have not seen it elsewhere.) If so, should we mention it in the Basicity section of this article as an alternative to the usual Bronsted definition of Kb for appropriate solutes. If not, should we delete all the Basicity values from the metal hydroxide infoboxes because they do not conform to the correct (Bronsted) definition of Kb? And what about the acidity value for Ca(OH)2 etc. Dirac66 (talk) 22:14, 17 December 2016 (UTC)
- The species Ca(O)OH- could occur in a very basic solvent such as liquid ammonia,
- Ca(OH)2 + NH3 ⇌Ca(O)(OH)- + NH4+
- but Ca(OH)2 only behaves as a base in aqueous solution. The two pK values are related to each other by pKa + pKb = pKW. When a value for one of the two constants is determined and reported in a publication there is uncertainty regarding the value of the other one unless the value of pKW, which depends on solvent, temperature and ionic strength, is specified; one value cannot be converted to the other willy nilly. (n.b.12.4 + 2.37 = 14.77 !) Petergans (talk) 10:03, 19 December 2016 (UTC)
- Hm. I had assumed that the pKa and pKb in the Ca(OH)2 infobox were both intended to describe Ca(OH)2. Whereas the relation pKa + pKb = pKW which you cite applies of course to an acid-base conjugate pair, and not to the pKa and pKb of a single species. I don't know which of us (if either) is interpreting the numbers correctly, and the ChemBuddy website whose URL I cited above claims that the pKb describes an Arrhenius-type dissociation of OH-. So now we have three interpretations - clearly we need the source of the numbers so we can verify which is correct. Dirac66 (talk) 19:58, 19 December 2016 (UTC)
- I have searched the literature using SC-database. The equilibrium
- Ca2+ + OH- ⇌ Ca(OH)+
- has a reported logKa values at 25C in the range 0.96 - 1.51 (17 publications) and logKb between 10.3 and 13.36 (3 publications). The log beta value for
- Ca2+ + 2OH- ⇌ Ca(OH)2
- has been estimated from solubility data at 3.14 (1 publication); this value is reasonable but has not been confirmed experimentally. Petergans (talk) 12:35, 20 December 2016 (UTC)
- OK, these Ka and Kb values tell us that some authors do use this notation for this non-Bronsted equilibrium. Can you find in the SC-database or elsewhere a clear statement of what reactions have these equilibrium constants? I could start guessing but it seems preferable to have a source. You have written that logKa and logKb refer to the same equilibrium which I do not understand.
- As for the beta value, does this refer to the β12 at Stability constants of complexes#Stepwise and cumulative constants? If so, does [ML2] refer to solid Ca(OH)2, in which case it seems β12 should be very simply related to solubility and I don't see why experimental confirmation is a problem? Or does it refer to dissolved neutral Ca(OH)2, which might be harder to measure? Dirac66 (talk) 20:40, 20 December 2016 (UTC)
- I have searched the literature using SC-database. The equilibrium
- Hm. I had assumed that the pKa and pKb in the Ca(OH)2 infobox were both intended to describe Ca(OH)2. Whereas the relation pKa + pKb = pKW which you cite applies of course to an acid-base conjugate pair, and not to the pKa and pKb of a single species. I don't know which of us (if either) is interpreting the numbers correctly, and the ChemBuddy website whose URL I cited above claims that the pKb describes an Arrhenius-type dissociation of OH-. So now we have three interpretations - clearly we need the source of the numbers so we can verify which is correct. Dirac66 (talk) 19:58, 19 December 2016 (UTC)
The following convention is generally understood, but should be stated explicitly in publications. For the general equilibrium (no electrical charges specified)
- pA + qB + rH ⇌ ApBqHr
the equilibrium constant is defined, in terms of the equilibrium concentrations, [A], etc (more rigorously, activities {A}...) by
- [ApBqHr] = βpqr[A]p[B]q[H]r
The only caveat is that this defines an association constant, with product on the left.For dissociation constants reagents and product swap positions. pKa is an acid dissociation constant, whereas pKb is a base association constant. The two concepts can be applied to the same protonation/deprotonation process, in which case the sum equates to pKw, as stated above.Petergans (talk) 10:53, 22 December 2016 (UTC)
- Yes, this definition is clear as long as the species in question are identified and well defined, which should be done in the article on each chemical species if there is any doubt. For protonation/deprotonation the species are usually clear to chemists, but perhaps not always to all Wikipedia readers.
- And the relation pKa + pKb = pKw is true provided pKa and pKb refer to a single acid-base conjugate pair, but then of course the two values do not describe the acidity and basicity of the same species as the infobox format might suggest if no notes are added. For example, the infobox for acetic acid says "Acidity pKa 4.76" and "Basicity pKb 9.24 (basicity of acetate ion)". The parenthetical note is there to help the reader who might otherwise imagine that 9.24 is the pKb of acetic acid !?? Similarly, the infobox for Bicarbonate says "Acidity (pKa) 10.3 (Conjugate acid of carbonate)" and "Basicity (pKb) 7.7 (Conjugate base of carbonic acid)". Here the reader might wonder why pKa + pKb is 18 and not 14, so the notes clarify that they do not refer to the same conjugate pair.
- For Ca(OH)2, pKa + pKb = 14.77 which is closer to 14. I assumed above that the values nevertheless refer to two different conjugate pairs (three species) as for HCO3-, whereas I think you assumed that they do refer to a single pair (species) and that the difference from 14 is due to some other effect: temperature? non-ideality? different experimental results? I don't know which is correct and I think we need a source which specifies the reactions involved. Then we could put a note in the infobox, as for acetic acid and bicarbonate ion. Dirac66 (talk) 19:27, 22 December 2016 (UTC)
- For the alkali metal hydroxides (MOH) which are simpler, one idea is a note in the infobox after the pKb values saying (Dissociation of OH-). However if the equilibrium is assumed to be MOH(s) = M+(aq) + OH-(aq), then (in ideal solution) Kb = Ksp = [M+][OH-] = s2. Unfortunately this fits the infobox data very poorly. For LiOH s = 128 g/L = 5.33 mol/L leading to Kb = 28.44 and pKb = -1.45, but the reported basicity is pKb = -0.36. Since pKb is a logarithmic scale, the error in Kb is greater than a factor of 10. Can this large an error be due to non-ideality, or have I used a wrong reaction for basicity? For NaOH the disagreement is worse; the solubility leads to pKb = -2.89 compared to the reported pKb =+0.2. And for CsOH the solubility leads to pKb = -2.60 and the reported pKb =-1.76. (For KOH and RbOH there are no pKb values in the infoboxes, although there is an acidity pKa 13.5 for KOH. If that means pKb = 14 - 13.5 = 0.5, it again disagrees with the value from solubility which is -2.67.) Dirac66 (talk) 02:25, 27 December 2016 (UTC)
I understand the points you are making. The only way to avoid ambiguity is to attach a definition to the property whose value is being quoted. Maybe there should be a separate page which describes the alternative definitions for pKa etc. with a link to that page attached to each numerical value in an infobox. Petergans (talk) 11:13, 27 December 2016 (UTC)
- Finally I have added a brief note with the alternative definition of Kb to this article. I will forget about the purported Ka values whose definition remains unclear to me, since as you say pKa + pKb does not seem to equal 14 as it should. Next I will change the infobox values and sources for the metal hydroxides. [Edit by Dirac66, 4 January 2017]
Metal ion hydrolysis
There are two complementary ways of defining a simple hydrolysis equilibrium
- Mn+ + OH- ⇌ M(OH)(n-1)+ : [M(OH)] = K[M][OH]
- Mn+ + H2O ⇌ M(OH)(n-1)+ + H+ : [M(OH)] = K*[M][H]-1
Both definitions (K and K star) are widely used in the literature. The vakues are related as follows
- KW=[H][OH]
- [M(OH)] =K[M][OH] = KKw[M][H]=1
- K*=KKW; log K* ≈ log K - 14
In general
- M + n(OH) ⇌ M(OH)n, logK* ≈ logK - 14n.
See also Metal_ions_in_aqueous_solution#Hydrolysis_of_aqua_ions Petergans (talk) 10:39, 7 January 2017 (UTC)
External links modified
Hello fellow Wikipedians,
I have just modified 6 external links on Acid dissociation constant. Please take a moment to review my edit. If you have any questions, or need the bot to ignore the links, or the page altogether, please visit this simple FaQ for additional information. I made the following changes:
- Added archive https://web.archive.org/web/20081029193538/http://www.iupac.org/web/ins/2000-003-1-500 to http://www.iupac.org/web/ins/2000-003-1-500
- Added archive https://web.archive.org/web/20081009060809/http://www.chem.wisc.edu/areas/reich/pkatable/ to http://www.chem.wisc.edu/areas/reich/pkatable/
- Added archive https://web.archive.org/web/20081006062140/http://www.nist.gov/data/PDFfiles/jpcrd615.pdf to http://www.nist.gov/data/PDFfiles/jpcrd615.pdf
- Corrected formatting/usage for http://sparc.chem.uga.edu/
- Corrected formatting/usage for http://jesuitnola.org/upload/clark/Refs/aqueous.htm
- Corrected formatting/usage for http://chemaxon.com/marvin/sketch/index.jsp
When you have finished reviewing my changes, you may follow the instructions on the template below to fix any issues with the URLs.
This message was posted before February 2018. After February 2018, "External links modified" talk page sections are no longer generated or monitored by InternetArchiveBot. No special action is required regarding these talk page notices, other than regular verification using the archive tool instructions below. Editors have permission to delete these "External links modified" talk page sections if they want to de-clutter talk pages, but see the RfC before doing mass systematic removals. This message is updated dynamically through the template {{source check}} (last update: 5 June 2024).
- If you have discovered URLs which were erroneously considered dead by the bot, you can report them with this tool.
- If you found an error with any archives or the URLs themselves, you can fix them with this tool.
Cheers.—InternetArchiveBot (Report bug) 02:17, 26 June 2017 (UTC)
Concentrated aqueous solutions of strong acids and pH-meters readings
It is not clear (and thus not specified in article) what is degree of dissociation of concentrated solutions of about 5-10 M which clearly is not 99-100%, but presumably much below/lower 70-85%. Also along with the unknown degree of dissociation goes the indication of pH-meters in these solutions, which needs to be clarified.--5.2.200.163 (talk) 10:05, 4 July 2018 (UTC)
Also the link between dissociation degree of concentrated strong acids, leveling effect, various acidity functions and pH-meters readings needs clarification.--5.2.200.163 (talk) 10:46, 4 July 2018 (UTC)
- The word "presumably" is not acceptable in Wikipedia. Please provide a citation to back up the assertion.
- Glass electrodes show deviations from ideal behaviour at very low pH, so cannot be used to measure hydrogen ion concentration without special calibration procedures.
- Ion-association must occur in a very concentrated solution when there are not enough water molecules to fully solvate the ions (see hydronium#solvation for details concerning the number of water molecules involved). In this article ion-association is implicitly assumed to be negligible.
Petergans (talk) 13:05, 4 July 2018 (UTC)
- The word presumably is just mentioned here on talk and not in article.--5.2.200.163 (talk) 15:42, 5 July 2018 (UTC)
- Interesting info about glass electrode.--5.2.200.163 (talk) 16:13, 5 July 2018 (UTC)
Threshold value of concentration needed for effectively complete ionic dissociation of strong acids (like hydrochloric)
The article in the subsection Strong acid and bases contains the following text:
A strong acid is effectively completely dissociated in aqueous solution. All acids with a pKa value of less than −2 are more than 99% dissociated at pH 0 (1 M acid). This is known as solvent leveling since such acids are brought to the same level of being strong acids, regardless of their pKa values. .... An example of a strong acid is hydrochloric acid, HCl, which has a pKa value, estimated from thermodynamic quantities, of −9.3 in water.[1] The concentration of undissociated HCl molecules in aqueous solution will be less than 0.01% of the analytical concentration of the acid. Hydrochloric acid is said to be fully dissociated in aqueous solution because the amount of undissociated acid, in equilibrium with the dissociation products, is below the detection limit.
The missing aspect is the threshold value of concentration where this assumption of complete dissociation starts not to hold, say above 1-2M (above 40 g/l), considering also the activity coefficients.--5.2.200.163 (talk) 14:19, 3 July 2018 (UTC)
- The extent of dissociation is a continuous function. For an acid of formula AH with a dissociation constant K, the concentration [H] of the dissociation products can be found by solving the quadratic equation
- TH = [H] + K[H]2
- where TH is the analytical concentration of the acid and [A] = [H]. Therefore, there is no specific threshold value. Empirically, an acid is classified as a strong acid when the concentration, in solution, of the undissociated species AH is too low to be determined by any known analytical technique. Petergans (talk) 15:19, 3 July 2018 (UTC)
- The above equation is simplistic, partly because of the absence of activity coefficients needed for concentrated solutions of strong acids like HCl, HNO3, H2SO4 of say 5-8 M. At these concentrations in aqueous solutions certainly these acids are not 99-100% dissociated, perhaps 75-80% dissociated.--5.2.200.163 (talk) 09:36, 4 July 2018 (UTC)
- For a hydrochloric acid solution of 5M, using the usual assumption of total dissociation, the calculated pH would be negative. The question is then: What would a pH-meter indicate in this mentioned solution? (I think that examples of individual data of concentrated solutions are useful.)--5.2.200.163 (talk) 09:43, 4 July 2018 (UTC)
- A link from ChemStackExchange which analyze the issue of 5M HCl https://chemistry.stackexchange.com/questions/28542/what-is-the-ph-of-a-5m-solution-of-hydrochloric-acid--5.2.200.163 (talk) 10:09, 4 July 2018 (UTC)
- I see that this link allows negative values of pH which are denied at Hammett acidity function.--5.2.200.163 (talk) 10:43, 4 July 2018 (UTC)
- The equation TH = [H] + K[H]2 is not simplistic - it is a statement of the law of conservation of mass. By definition, an equilibrium constant is independent of concentration. If ion-association were to occur, forming a 1:1 complex, the equation will become
- TH = [H] + (K+Kassociation)[H]2
- ChemStackExchange is not an acceptable source to verify statements - see Wikipedia:Verifiability Petergans (talk) 13:28, 4 July 2018 (UTC)
- Of course CSE is as you say, that′s why is just mentioned here on a talk page (for estimation/approximation purposes) and not in article.--5.2.200.163 (talk) 15:40, 5 July 2018 (UTC)
- The equation TH = [H] + K[H]2 is not simplistic - it is a statement of the law of conservation of mass. By definition, an equilibrium constant is independent of concentration. If ion-association were to occur, forming a 1:1 complex, the equation will become
References
- ^ Dasent, W.E. (1982). Inorganic Energetics: An Introduction. Cambridge University Press. ISBN 0-521-28406-6. Chapter 5
Equilibrium defined as not changing in time
The textbook by Whitten et al. was added yesterday as a reference for the statement that chemical species are in equilibrium when their concentrations do not change with time. But I now have the cited page (708) in front of me and I see nothing about concentration of solute species changing with time, so there seems to be a citation error here.
In fact this definition is dubious since it could be understood to describe reactions which are very slow. Diamond in contact with graphite appears not to change at a measurable rate, but we know that they are not really in equilibrium.
A better simple definition is found on p.660-1 of the same textbook at the beginning of the chapter in terms of dynamic equilibrium: chemical equilibrium exists when two opposing reactions occur simultaneously at the same rate. Dirac66 (talk) 12:15, 18 February 2019 (UTC)
- The reference to Whitten is not new: I kept it in the lead in good faith, but was not able to check it. The quote above has now been incorporated in the lead text. Petergans (talk) 15:18, 18 February 2019 (UTC)
- OK, I see what happened. Whitten was inserted last June 7 by me as a source for the statement that [H2O] is omitted because [H2O] is essentially constant. In yesterday's rewrite you deleted that fact to the intro, and used Whitten as a reference for all concentrations being constant, which is not what p.708 says. However the new version with Whitten p.660 as a source for dynamic equilibrium is much better. Dirac66 (talk) 17:36, 18 February 2019 (UTC)


![{\displaystyle K^{\ominus }={{\frac {[c({\rm {A^{-}}})/c^{\ominus }][c({\rm {H_{3}O^{+}}})/c^{\ominus }]}{[c({\rm {HA}})/c^{\ominus }][c({\rm {H_{2}O}})/c^{\ominus }]}}\times {\frac {\gamma _{\rm {A^{-}}}\gamma _{\rm {H_{3}O^{+}}}}{\gamma _{\rm {HA}}\gamma _{\rm {H_{2}O}}}}=\mathrm {\frac {[A^{-}][H_{3}O^{+}]}{[HA][H_{2}O]}} }\times \Gamma }](https://wikimedia.org/api/rest_v1/media/math/render/svg/1404da3344135ff5039add6bf1f0e62aa14b3219)

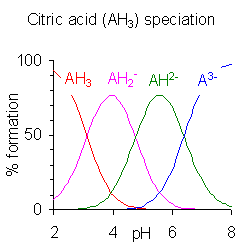
![{\displaystyle \alpha _{H_{n-i}A^{i-}}={{[H^{+}]^{n-i}\displaystyle \prod _{j=0}^{i}K_{j}} \over {\displaystyle \sum _{i=0}^{n}{\Big [}[H^{+}]^{n-i}\displaystyle \prod _{j=0}^{i}K_{j}}{\Big ]}}}](https://wikimedia.org/api/rest_v1/media/math/render/svg/04ba12927aeedcbc8146e405ddef74aa7cfa343c)
![{\displaystyle \alpha _{H_{2}A}={{[H^{+}]^{2}} \over {[H^{+}]^{2}+[H^{+}]K_{1}+K_{1}K_{2}}}}](https://wikimedia.org/api/rest_v1/media/math/render/svg/6c83788df1aff4ef4cbd1bb8a7e99904cecf8737)
![{\displaystyle \alpha _{HA^{-}}={{[H^{+}]K_{1}} \over {[H^{+}]^{2}+[H^{+}]K_{1}+K_{1}K_{2}}}}](https://wikimedia.org/api/rest_v1/media/math/render/svg/5652796bc022d89f07ae3822c98864a338e95616)
![{\displaystyle \alpha _{A^{2-}}={{K_{1}K_{2}} \over {[H^{+}]^{2}+[H^{+}]K_{1}+K_{1}K_{2}}}}](https://wikimedia.org/api/rest_v1/media/math/render/svg/fb696c42def57f8b73201a30eead67c48f367208)

![{\displaystyle K_{a}={\frac {[A^{-}][H^{+}]}{[HA]}}}](https://wikimedia.org/api/rest_v1/media/math/render/svg/a3064f673dfe4e209bbc32b2fa3be27574f498c6)
![{\displaystyle [H^{+}]={\sqrt {[HA][K_{a}]}}}](https://wikimedia.org/api/rest_v1/media/math/render/svg/d497573f42e78f6933688d5f527a52e2bb4d8083)


![{\displaystyle K={\frac {[HA]}{[H^{+}][A^{-}]}}}](https://wikimedia.org/api/rest_v1/media/math/render/svg/a11cc1b8bc43f42968edce347be8d451b0f0352f)
![{\displaystyle K={\frac {[H^{+}][A^{-}]}{[HA]}}}](https://wikimedia.org/api/rest_v1/media/math/render/svg/17ce0953b2b65c687c7eedbccd8f1ea5728a3a3c)