Talk:Acetylacetone
| This article is rated B-class on Wikipedia's content assessment scale. It is of interest to the following WikiProjects: | |||||||||||
| |||||||||||
| Text and/or other creative content from this version of Acetylacetone was copied or moved into Metal_acetylacetonates with this edit. The former page's history now serves to provide attribution for that content in the latter page, and it must not be deleted as long as the latter page exists. |
Structure
[edit]The structure of the enol indicates that it is symmetric but I suspect that it is not. Also, I think that the enol form has been isolated free of the diketo, amazingly. --Smokefoot 15:28, 14 April 2006 (UTC)
- Well the reference for the C2v (ie, symmetrical) structure of the enol is given in the article... You probably have better access to JACS than I do (especially as I'm about to head off on vacation :), so I should look up the article "and references therein". Physchim62 (talk) 15:40, 14 April 2006 (UTC)
Preparation
[edit]Is the reaction for preparation similar to the aldol reaction?
- Yes, it is similar. It is also related to a Claisen condensation. All these reactions involve the attack of an enolate at a carbonyl carbon. Physchim62 (talk) 10:49, 18 May 2006 (UTC)
Calcium acetylacetonate
[edit]An important industrial application of acetylacetone is the calcium complex which is a useful thermal stabiliser for PVC resins and compounds. (57.67.16.50 10:56, 4 January 2007 (UTC))
Zinc acac
[edit]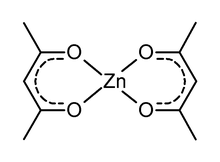
I was replacing jpg images made User:J. Atkinson and I replaced the image of zinc acac with image:Zn acac.png. But, it is not mentioned in the text, so I am leaving it here in case anyone needs it. --Rifleman 82 03:04, 13 October 2007 (UTC)
- It is worth checking, but Zn(acac)2 might not be square planar or even monomeric when anhydrous, and probably the usual form is hydrated with water ligand(s). But I dont know for sure. --Smokefoot 04:04, 13 October 2007 (UTC)
Hmm... I think you've found out. It looks like this image won't give a good representation of the hydrated molecule. What about the anhydrous? I can always delete this molecule if it doesn't fit. --Rifleman 82 03:47, 14 October 2007 (UTC)
- I couldnt find the structure of the anhydrous in a quick search. I'd keep the image because it might be right.--Smokefoot 03:50, 14 October 2007 (UTC)
- In any case, I think it is often acceptable to leave out solvents of crystallization, esp. in 2D structures which emphasize connectivity than 3D structure. --Rifleman 82 03:56, 14 October 2007 (UTC)
Clarification needed on K
[edit]Just to make it easier for someone to fix this, I will repeat my description of the problem (from the edit summary that marked it). The problem is that the directionality of the equation for which the K is given is very important, and, while it is somewhat implicit which direction that is based on the first sentence of the paragraph, it really needs to be *explicit*, so that it would say "The K is 11.7 for the equilibrium of the keto to enol, as shown below:" Then have a diagram showing the equation, since there arent any plaintext characters that can represent the double arrows used in equilibrium equations. Thanks in advance to anyone who addresses this or even just attempts to -- my chemistry just isn't good enough, nor are my skills at searching the internet for such info. Mr0t1633 (talk) 18:44, 21 January 2010 (UTC)
- Just checked to make sure I wasn't missing anything and a fitting example of why this is necessary was right in front of me: the first sentence mentioned above uses 'keto' before 'enol' immediately before making reference to the equilibrium value, then the values in different solvents. However, the diagram at the bottom shows the enol first, then the keto. So which is it? Mr0t1633 (talk) 18:47, 21 January 2010 (UTC)
tautomers
[edit]
This structural diagram is misleading. The central carbon atom in the enol form has one hydrogen attached, not two. This needs to be shown explicitly. Petergans (talk) 16:51, 18 January 2019 (UTC)

