Sommelet reaction
The Sommelet reaction is an organic reaction in which a benzyl halide is converted to an aldehyde by action of hexamine and water.[1][2] It is named after the French chemist Marcel Sommelet, who first reported the reaction in 1913.[3]
One example, thiophene-2-carboxaldehyde is prepared by the reaction of hexamine with 2-chloromethylthiophene.[4] The reaction is formally an oxidation of the carbon.
Reaction mechanism and scope
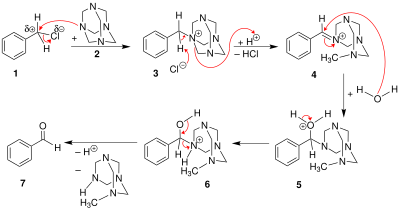
[edit]The benzyl halide 1 reacts with hexamine to a quaternary ammonium salt 3, each time just alkylating one nitrogen atom. Then the benzylammonium undergoes an acid-catalyzed hydrolysis process.
Depending on the hydrolysis conditions, the hexamine unit might instead break apart, leaving a benzyl amine (the Delépine reaction).
The reaction can also be applied to the oxidation of benzylic amines. In this way, m-xylylenediamine can be converted to isophthalaldehyde.[5]
References
[edit]- ^ March, Jerry (1985), Advanced Organic Chemistry: Reactions, Mechanisms, and Structure, 3rd edition, New York: Wiley, ISBN 9780471854722, OCLC 642506595
- ^ Angyal, S. J. (15 March 2011). "The Sommelet Reaction". Organic Reactions: 197–217. doi:10.1002/0471264180.or008.04. ISBN 978-0-471-26418-7.
- ^ Marcel Sommelet (1913). "Sur un mode de décomposition des halogénoalcoylates d'hexaméthylène – tétramine". Compt. Rend. 157: 852–854.
- ^ Kenneth B. Wiberg. "2-Thiophenealdehyde". Org. Synth. 3: 811. doi:10.15227/orgsyn.000.0005.
- ^ Ackerman, J. H.; Surrey, A. R. (1967). "Isophthalaldehyde". Organic Syntheses. 47: 76. doi:10.15227/orgsyn.047.0076.