Tin(IV) bromide
Appearance
(Redirected from SnBr4)

| |
| |
| Names | |
|---|---|
| IUPAC name
tetrabromostannate
| |
| Other names
tin tetrabromide, stannic bromide, bromostannic acid
| |
| Identifiers | |
3D model (JSmol)
|
|
| ChemSpider | |
| ECHA InfoCard | 100.029.258 |
| EC Number |
|
PubChem CID
|
|
| UNII | |
CompTox Dashboard (EPA)
|
|
| |
| |
| Properties | |
| SnBr4 | |
| Molar mass | 438.33 g/mol |
| Appearance | colourless [1] |
| Density | 3.340 g/cm3 (at 35 °C)[1] |
| Melting point | 31 °C (88 °F; 304 K)[1] |
| Boiling point | 205 °C (401 °F; 478 K)[1] |
| soluble | |
| −149.0·10−6 cm3/mol | |
| Related compounds | |
Other anions
|
Tin(IV) fluoride Tin(IV) chloride Tin(IV) iodide |
Other cations
|
Carbon tetrabromide Silicon tetrabromide Germanium tetrabromide |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
| |
Tin(IV) bromide is the chemical compound SnBr4. It is a colourless low melting solid.[1]
Structure
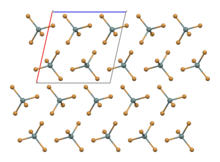
[edit]SnBr4 occurs in form of crystals. The compound crystallises in a monoclinic crystal system with molecular SnBr4 units that have distorted tetrahedral geometry,[2] with mean Sn-Br bond lengths of 242.3 pm.[3]
Preparation
[edit]SnBr4 can be prepared by reaction of the elements at standard temperature and pressure (STP):[4][page needed]
- Sn + 2Br
2 → SnBr
4
Dissolution in solvents
[edit]In aqueous solution SnBr4 dissolves to give a series of octahedral (six-ligated) bromo-aquo complexes. These include SnBr4(H2O)2 and cis- and trans-[SnBr2(H2O)4]2+.[5]
Reactions
[edit]SnBr4 forms 1:1 and 1:2 complexes with ligands, e.g. with trimethylphosphine the following can be produced, SnBr4.P(CH3)3 and SnBr4.2P(CH3)3.[6]
References
[edit]- ^ a b c d e Greenwood, Norman N.; Earnshaw, Alan (1997). Chemistry of the Elements (2nd ed.). Butterworth-Heinemann. ISBN 978-0-08-037941-8.
- ^ Brand, P.; Sackmann, H. (1963). "Die Kristallstruktur von SnBr4" [The crystal structure of SnBr4]. Acta Crystallographica (in German). 16 (6): 446–451. Bibcode:1963AcCry..16..446B. doi:10.1107/S0365110X63001250.
- ^ Reuter, H.; Pawlak, R. (2001). "Zinnhalogenverbindungen. II. Die Molekül- und Kristallstrukturen von Zinn(IV)-bromid und -iodid" [Tin halogen compounds. II. The Molecular and Crystal Structures of Tin(IV) Bromide and Tin(IV) Iodide]. Zeitschrift für Kristallographie – Crystalline Materials [Journal of Crystallography - Crystalline Materials] (in German). 216 (1–2001): 34–38. Bibcode:2001ZK....216...34R. doi:10.1524/zkri.216.1.34.18992. S2CID 94609783.
- ^ Wiberg, Egon; Wiberg, Nils; Holleman, Arnold Frederick (2001). Inorganic Chemistry. Academic Press, Elsevier. ISBN 978-0-12-352651-9. OCLC 1024925228.
- ^ Taylor, M. J.; Coddington, J. M. (1992). "The constitution of aqueous tin(IV) chloride and bromide solutions and solvent extracts studied by 119Sn NMR and vibrational spectroscopy". Polyhedron. 11 (12): 1531–1544. doi:10.1016/S0277-5387(00)83148-4.
- ^ Frieson, D. K.; Ozin, G. A. (1973). "Preparation, Infrared and Raman Spectra, and Stereochemistries of Pentacoordinate Trimethylphosphine Complexes, MX4•P(CH3)3 and MX4•P(CD3)3 where M = Ge or Sn and X = Cl or Br". Canadian Journal of Chemistry. 51 (16): 2697–2709. doi:10.1139/v73-406.
