Habitat



In ecology, habitat refers to the array of resources, physical and biotic factors that are present in an area, such as to support the survival and reproduction of a particular species. A species habitat can be seen as the physical manifestation of its ecological niche. Thus "habitat" is a species-specific term, fundamentally different from concepts such as environment or vegetation assemblages, for which the term "habitat-type" is more appropriate.[2]
The physical factors may include (for example): soil, moisture, range of temperature, and light intensity. Biotic factors include the availability of food and the presence or absence of predators. Every species has particular habitat requirements, habitat generalist species are able to thrive in a wide array of environmental conditions while habitat specialist species require a very limited set of factors to survive. The habitat of a species is not necessarily found in a geographical area, it can be the interior of a stem, a rotten log, a rock or a clump of moss; a parasitic organism has as its habitat the body of its host, part of the host's body (such as the digestive tract), or a single cell within the host's body.[3]
Habitat types are environmental categorizations of different environments based on the characteristics of a given geographical area, particularly vegetation and climate.[2] Thus habitat types do not refer to a single species but to multiple species living in the same area. For example, terrestrial habitat types include forest, steppe, grassland, semi-arid or desert. Fresh-water habitat types include marshes, streams, rivers, lakes, and ponds; marine habitat types include salt marshes, the coast, the intertidal zone, estuaries, reefs, bays, the open sea, the sea bed, deep water and submarine vents.
Habitat types may change over time. Causes of change may include a violent event (such as the eruption of a volcano, an earthquake, a tsunami, a wildfire or a change in oceanic currents); or change may occur more gradually over millennia with alterations in the climate, as ice sheets and glaciers advance and retreat, and as different weather patterns bring changes of precipitation and solar radiation. Other changes come as a direct result of human activities, such as deforestation, the plowing of ancient grasslands, the diversion and damming of rivers, the draining of marshland and the dredging of the seabed. The introduction of alien species can have a devastating effect on native wildlife – through increased predation, through competition for resources or through the introduction of pests and diseases to which the indigenous species have no immunity.
Definition and etymology
[edit]The word "habitat" has been in use since about 1755 and derives from the Latin habitāre, to inhabit, from habēre, to have or to hold. Habitat can be defined as the natural environment of an organism, the type of place in which it is natural for it to live and grow.[4][5] It is similar in meaning to a biotope; an area of uniform environmental conditions associated with a particular community of plants and animals.[6]
Environmental factors
[edit]The chief environmental factors affecting the distribution of living organisms are temperature, humidity, climate, soil and light intensity, and the presence or absence of all the requirements that the organism needs to sustain it. Generally speaking, animal communities are reliant on specific types of plant communities.[7]
Some plants and animals have habitat requirements which are met in a wide range of locations. The small white butterfly Pieris rapae for example is found on all the continents of the world apart from Antarctica. Its larvae feed on a wide range of Brassicas and various other plant species, and it thrives in any open location with diverse plant associations.[8] The large blue butterfly Phengaris arion is much more specific in its requirements; it is found only in chalk grassland areas, its larvae feed on Thymus species, and because of complex life cycle requirements it inhabits only areas in which Myrmica ants live.[9]
Disturbance is important in the creation of biodiverse habitat types. In the absence of disturbance, a climax vegetation cover develops that prevents the establishment of other species. Wildflower meadows are sometimes created by conservationists but most of the flowering plants used are either annuals or biennials and disappear after a few years in the absence of patches of bare ground on which their seedlings can grow.[10] Lightning strikes and toppled trees in tropical forests allow species richness to be maintained as pioneering species move in to fill the gaps created.[11] Similarly, coastal habitat types can become dominated by kelp until the seabed is disturbed by a storm and the algae swept away, or shifting sediment exposes new areas for colonisation. Another cause of disturbance is when an area may be overwhelmed by an invasive introduced species which is not kept under control by natural enemies in its new habitat.[12]
Types
[edit]Terrestrial
[edit]
Terrestrial habitat types include forests, grasslands, wetlands and deserts. Within these broad biomes are more specific habitat types with varying climate types, temperature regimes, soils, altitudes and vegetation. Many of these habitat types grade into each other and each one has its own typical communities of plants and animals. A habitat-type may suit a particular species well, but its presence or absence at any particular location depends to some extent on chance, on its dispersal abilities and its efficiency as a colonizer.[13]
Arid
[edit]
Arid habitats are those where there is little available water. The most extreme arid habitats are deserts. Desert animals have a variety of adaptations to survive the dry conditions. Some frogs live in deserts, creating moist habitat types underground and hibernating while conditions are adverse. Couch's spadefoot toad (Scaphiopus couchii) emerges from its burrow when a downpour occurs and lays its eggs in the transient pools that form; the tadpoles develop with great rapidity, sometimes in as little as nine days, undergo metamorphosis, and feed voraciously before digging a burrow of their own.[14]
List of arid habitat types
[edit]Wetland and riparian
[edit]Other organisms cope with the drying up of their aqueous habitat in other ways. Vernal pools are ephemeral ponds that form in the rainy season and dry up afterwards. They have their specially-adapted characteristic flora, mainly consisting of annuals, the seeds of which survive the drought, but also some uniquely adapted perennials.[15] Animals adapted to these extreme habitat types also exist; fairy shrimps can lay "winter eggs" which are resistant to desiccation, sometimes being blown about with the dust, ending up in new depressions in the ground. These can survive in a dormant state for as long as fifteen years.[16] Some killifish behave in a similar way; their eggs hatch and the juvenile fish grow with great rapidity when the conditions are right, but the whole population of fish may end up as eggs in diapause in the dried up mud that was once a pond.[17]
Examples of wetland and riparian habitat types
[edit]Forest
[edit]Examples of forest habitat types
[edit]- Boreal forest
- Cloud forest
- Peat swamp forest
- Temperate coniferous forest
- Temperate deciduous forest
- Temperate rain forest
- Thorn forest
- Tropical dry forest
- Tropical moist forest
- Tropical rain forest
- Woodland
Freshwater
[edit]
Freshwater habitat types include rivers, streams, lakes, ponds, marshes and bogs.[18] They can be divided into running waters (rivers, streams) and standing waters (lakes, ponds, marshes, bogs).[19] Although some organisms are found across most of these habitat types, the majority have more specific requirements. The water velocity, its temperature and oxygen saturation are important factors, but in river systems, there are fast and slow sections, pools, bayous and backwaters which provide a range of habitat types. Similarly, aquatic plants can be floating, semi-submerged, submerged or grow in permanently or temporarily saturated soils besides bodies of water. Marginal plants provide important habitat for both invertebrates and vertebrates, and submerged plants provide oxygenation of the water, absorb nutrients and play a part in the reduction of pollution.[20]
List of freshwater habitat types
[edit]Rivers Ponds Lakes
Marine
[edit]Marine habitats include brackish water, estuaries, bays, the open sea, the intertidal zone, the sea bed, reefs and deep / shallow water zones.[18] Further variations include rock pools, sand banks, mudflats, brackish lagoons, sandy and pebbly beaches, and seagrass beds, all supporting their own flora and fauna. The benthic zone or seabed provides a home for both static organisms, anchored to the substrate, and for a large range of organisms crawling on or burrowing into the surface. Some creatures float among the waves on the surface of the water, or raft on floating debris, others swim at a range of depths, including organisms in the demersal zone close to the seabed, and myriads of organisms drift with the currents and form the plankton.[21]
List of marine habitat types
[edit]Urban
[edit]Many animals and plants have taken up residence in urban environments. They tend to be adaptable generalists and use the town's features to make their homes. Rats and mice have followed man around the globe, pigeons, peregrines, sparrows, swallows and house martins use the buildings for nesting, bats use roof space for roosting, foxes visit the garbage bins and squirrels, coyotes, raccoons and skunks roam the streets. About 2,000 coyotes are thought to live in and around Chicago.[22] A survey of dwelling houses in northern European cities in the twentieth century found about 175 species of invertebrate inside them, including 53 species of beetle, 21 flies, 13 butterflies and moths, 13 mites, 9 lice, 7 bees, 5 wasps, 5 cockroaches, 5 spiders, 4 ants and a number of other groups.[23] In warmer climates, termites are serious pests in the urban habitat; 183 species are known to affect buildings and 83 species cause serious structural damage.[24]
Microhabitat types
[edit]
A microhabitat is the small-scale physical requirements of a particular organism or population. Every habitat includes large numbers of microhabitat types with subtly different exposure to light, humidity, temperature, air movement, and other factors. The lichens that grow on the north face of a boulder are different from those that grow on the south face, from those on the level top, and those that grow on the ground nearby; the lichens growing in the grooves and on the raised surfaces are different from those growing on the veins of quartz. Lurking among these miniature "forests" are the microfauna, species of invertebrate, each with its own specific habitat requirements.[25]
There are numerous different microhabitat types in a wood; coniferous forest, broad-leafed forest, open woodland, scattered trees, woodland verges, clearings, and glades; tree trunk, branch, twig, bud, leaf, flower, and fruit; rough bark, smooth bark, damaged bark, rotten wood, hollow, groove, and hole; canopy, shrub layer, plant layer, leaf litter, and soil; buttress root, stump, fallen log, stem base, grass tussock, fungus, fern, and moss.[26] The greater the structural diversity in the wood, the greater the number of microhabitat types that will be present. A range of tree species with individual specimens of varying sizes and ages, and a range of features such as streams, level areas, slopes, tracks, clearings, and felled areas will provide suitable conditions for an enormous number of biodiverse plants and animals. For example, in Britain it has been estimated that various types of rotting wood are home to over 1700 species of invertebrate.[26]
For a parasitic organism, its habitat is the particular part of the outside or inside of its host on or in which it is adapted to live. The life cycle of some parasites involves several different host species, as well as free-living life stages, sometimes within vastly different microhabitat types.[27] One such organism is the trematode (flatworm) Microphallus turgidus, present in brackish water marshes in the southeastern United States. Its first intermediate host is a snail and the second, a glass shrimp. The final host is the waterfowl or mammal that consumes the shrimp.[28]
Extreme habitat types
[edit]
Although the vast majority of life on Earth lives in mesophyllic (moderate) environments, a few organisms, most of them microbes, have managed to colonise extreme environments that are unsuitable for more complex life forms. There are bacteria, for example, living in Lake Whillans, half a mile below the ice of Antarctica; in the absence of sunlight, they must rely on organic material from elsewhere, perhaps decaying matter from glacier melt water or minerals from the underlying rock.[29] Other bacteria can be found in abundance in the Mariana Trench, the deepest place in the ocean and on Earth; marine snow drifts down from the surface layers of the sea and accumulates in this undersea valley, providing nourishment for an extensive community of bacteria.[30]
Other microbes live in environments lacking in oxygen, and are dependent on chemical reactions other than photosynthesis. Boreholes drilled 300 m (1,000 ft) into the rocky seabed have found microbial communities apparently based on the products of reactions between water and the constituents of rocks. These communities have not been studied much, but may be an important part of the global carbon cycle.[31] Rock in mines two miles deep also harbour microbes; these live on minute traces of hydrogen produced in slow oxidizing reactions inside the rock. These metabolic reactions allow life to exist in places with no oxygen or light, an environment that had previously been thought to be devoid of life.[32][33]
The intertidal zone and the photic zone in the oceans are relatively familiar habitat types. However the vast bulk of the ocean is inhospitable to air-breathing humans, with scuba divers limited to the upper 50 m (160 ft) or so.[34] The lower limit for photosynthesis is 100 to 200 m (330 to 660 ft) and below that depth the prevailing conditions include total darkness, high pressure, little oxygen (in some places), scarce food resources and extreme cold. This habitat is very challenging to research, and as well as being little-studied, it is vast, with 79% of the Earth's biosphere being at depths greater than 1,000 m (3,300 ft).[35] With no plant life, the animals in this zone are either detritivores, reliant on food drifting down from surface layers, or they are predators, feeding on each other. Some organisms are pelagic, swimming or drifting in mid-ocean, while others are benthic, living on or near the seabed. Their growth rates and metabolisms tend to be slow, their eyes may be very large to detect what little illumination there is, or they may be blind and rely on other sensory inputs. A number of deep sea creatures are bioluminescent; this serves a variety of functions including predation, protection and social recognition.[35] In general, the bodies of animals living at great depths are adapted to high pressure environments by having pressure-resistant biomolecules and small organic molecules present in their cells known as piezolytes, which give the proteins the flexibility they need. There are also unsaturated fats in their membranes which prevent them from solidifying at low temperatures.[36]

Hydrothermal vents were first discovered in the ocean depths in 1977.[37] They result from seawater becoming heated after seeping through cracks to places where hot magma is close to the seabed. The under-water hot springs may gush forth at temperatures of over 340 °C (640 °F) and support unique communities of organisms in their immediate vicinity.[37] The basis for this teeming life is chemosynthesis, a process by which microbes convert such substances as hydrogen sulfide or ammonia into organic molecules.[38] These bacteria and Archaea are the primary producers in these ecosystems and support a diverse array of life. About 350 species of organism, dominated by molluscs, polychaete worms and crustaceans, had been discovered around hydrothermal vents by the end of the twentieth century, most of them being new to science and endemic to these habitat types.[39]
Besides providing locomotion opportunities for winged animals and a conduit for the dispersal of pollen grains, spores and seeds, the atmosphere can be considered to be a habitat-type in its own right. There are metabolically active microbes present that actively reproduce and spend their whole existence airborne, with hundreds of thousands of individual organisms estimated to be present in a cubic meter of air. The airborne microbial community may be as diverse as that found in soil or other terrestrial environments, however, these organisms are not evenly distributed, their densities varying spatially with altitude and environmental conditions. Aerobiology has not been studied much, but there is evidence of nitrogen fixation in clouds, and less clear evidence of carbon cycling, both facilitated by microbial activity.[40]
There are other examples of extreme habitat types where specially adapted lifeforms exist; tar pits teeming with microbial life;[41] naturally occurring crude oil pools inhabited by the larvae of the petroleum fly;[42] hot springs where the temperature may be as high as 71 °C (160 °F) and cyanobacteria create microbial mats;[43] cold seeps where the methane and hydrogen sulfide issue from the ocean floor and support microbes and higher animals such as mussels which form symbiotic associations with these anaerobic organisms;[44] salt pans that harbour salt-tolerant bacteria, archaea and also fungi such as the black yeast Hortaea werneckii and basidiomycete Wallemia ichthyophaga;[45][46] ice sheets in Antarctica which support fungi Thelebolus spp.,[45] glacial ice with a variety of bacteria and fungi;[47] and snowfields on which algae grow.[48]
Habitat change
[edit]
Whether from natural processes or the activities of man, landscapes and their associated habitat types change over time. There are the slow geomorphological changes associated with the geologic processes that cause tectonic uplift and subsidence, and the more rapid changes associated with earthquakes, landslides, storms, flooding, wildfires, coastal erosion, deforestation and changes in land use.[49] Then there are the changes in habitat types brought on by alterations in farming practices, tourism, pollution, fragmentation and climate change.[50]
Loss of habitat is the single greatest threat to any species. If an island on which an endemic organism lives becomes uninhabitable for some reason, the species will become extinct. Any type of habitat surrounded by a different habitat is in a similar situation to an island. If a forest is divided into parts by logging, with strips of cleared land separating woodland blocks, and the distances between the remaining fragments exceeds the distance an individual animal is able to travel, that species becomes especially vulnerable. Small populations generally lack genetic diversity and may be threatened by increased predation, increased competition, disease and unexpected catastrophe.[50] At the edge of each forest fragment, increased light encourages secondary growth of fast-growing species and old growth trees are more vulnerable to logging as access is improved. The birds that nest in their crevices, the epiphytes that hang from their branches and the invertebrates in the leaf litter are all adversely affected and biodiversity is reduced.[50] Habitat fragmentation can be ameliorated to some extent by the provision of wildlife corridors connecting the fragments. These can be a river, ditch, strip of trees, hedgerow or even an underpass to a highway. Without the corridors, seeds cannot disperse and animals, especially small ones, cannot travel through the hostile territory, putting populations at greater risk of local extinction.[51]
Habitat disturbance can have long-lasting effects on the environment. Bromus tectorum is a vigorous grass from Europe which has been introduced to the United States where it has become invasive. It is highly adapted to fire, producing large amounts of flammable detritus and increasing the frequency and intensity of wildfires. In areas where it has become established, it has altered the local fire regimen to such an extant that native plants cannot survive the frequent fires, allowing it to become even more dominant.[52] A marine example is when sea urchin populations "explode" in coastal waters and destroy all the macroalgae present. What was previously a kelp forest becomes an urchin barren that may last for years and this can have a profound effect on the food chain. Removal of the sea urchins, by disease for example, can result in the seaweed returning, with an over-abundance of fast-growing kelp.[53]
Fragmentation
[edit]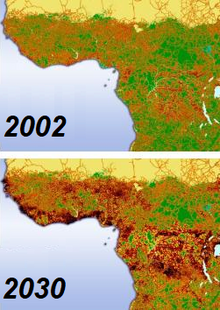


Destruction
[edit]
Habitat destruction (also termed habitat loss and habitat reduction) occurs when a natural habitat is no longer able to support its native species. The organisms once living there have either moved to elsewhere or are dead, leading to a decrease in biodiversity and species numbers.[59][60] Habitat destruction is in fact the leading cause of biodiversity loss and species extinction worldwide.[61]
Humans contribute to habitat destruction through the use of natural resources, agriculture, industrial production and urbanization (urban sprawl). Other activities include mining, logging and trawling. Environmental factors can contribute to habitat destruction more indirectly. Geological processes, climate change,[60] introduction of invasive species, ecosystem nutrient depletion, water and noise pollution are some examples. Loss of habitat can be preceded by an initial habitat fragmentation. Fragmentation and loss of habitat have become one of the most important topics of research in ecology as they are major threats to the survival of endangered species.[62]Habitat protection
[edit]The protection of habitat types is a necessary step in the maintenance of biodiversity because if habitat destruction occurs, the animals and plants reliant on that habitat suffer. Many countries have enacted legislation to protect their wildlife. This may take the form of the setting up of national parks, forest reserves and wildlife reserves, or it may restrict the activities of humans with the objective of benefiting wildlife. The laws may be designed to protect a particular species or group of species, or the legislation may prohibit such activities as the collecting of bird eggs, the hunting of animals or the removal of plants. A general law on the protection of habitat types may be more difficult to implement than a site specific requirement. A concept introduced in the United States in 1973 involves protecting the critical habitat of endangered species, and a similar concept has been incorporated into some Australian legislation.[63]
International treaties may be necessary for such objectives as the setting up of marine reserves. Another international agreement, the Convention on the Conservation of Migratory Species of Wild Animals, protects animals that migrate across the globe and need protection in more than one country.[64] Even where legislation protects the environment, a lack of enforcement often prevents effective protection. However, the protection of habitat types needs to take into account the needs of the local residents for food, fuel and other resources. Faced with hunger and destitution, a farmer is likely to plough up a level patch of ground despite it being the last suitable habitat for an endangered species such as the San Quintin kangaroo rat, and even kill the animal as a pest.[65] In the interests of ecotourism it is desirable that local communities are educated on the uniqueness of their flora and fauna.[66]
Monotypic habitat
[edit]A monotypic habitat type is a concept sometimes used in conservation biology, in which a single species of animal or plant is the only species of its type to be found in a specific habitat and forms a monoculture. Even though it might seem such a habitat type is impoverished in biodiversity as compared with polytypic habitat types, this is not necessarily the case. Monocultures of the exotic plant Hydrilla support a similarly rich fauna of invertebrates as a more varied habitat.[67] The monotypic habitat occurs in both botanical and zoological contexts. Some invasive species may create monocultural stands that prevent other species from growing there. A dominant colonization can occur from retardant chemicals exuded, nutrient monopolization, or from lack of natural controls, such as herbivores or climate, that keep them in balance with their native habitat types. The yellow starthistle, Centaurea solstitialis is a botanical monotypic habitat example of this, currently dominating over 15,000,000 acres (61,000 km2) in California alone.[68] The non-native freshwater zebra mussel, Dreissena polymorpha, that colonizes areas of the Great Lakes and the Mississippi River watershed, is a zoological monotypic habitat example; the predators or parasites that control it in its home-range in Russia are absent.[69]
See also
[edit]- Altitudinal zonation – Natural layering of ecosystems by elevation
- Ecological niche – Fit of a species living under specific environmental conditions
- Habitat conservation – Management practice for protecting types of environments
- Habitat destruction – Process by which a natural habitat becomes incapable of supporting its native species: the loss of habitat
- Habitat fragmentation – Discontinuities in an organism's environment causing population fragmentation.
- Landscape ecology – Science of relationships between ecological processes in the environment and particular ecosystems
- List of life zones by region
Notes and references
[edit]- ^ Wohlschlag, Donald E. (1968). "Fishes beneath Antarctic ice" (PDF). Australian Natural History. 16. Australian Museum: 45–48. Archived (PDF) from the original on 12 April 2021. Retrieved 19 July 2021.
Beneath the sea ice and the terminal portions of the Ross Ice Shelf and Koettlitz Glacier tongue is an aquatic habitat that has a nearly uniform freezing temperature of -1.9°C (28.6°F) and a remarkable assemblage of animals.
- ^ a b Krausman, Paul R.; Morrison, Michael L. (26 July 2016). "Another plea for standard terminology: Editor's Message". The Journal of Wildlife Management. 80 (7): 1143–1144. doi:10.1002/jwmg.21121.
- ^ For example:
Swapan Kumar Nath; Revankar, Sanjay G. (2006). Problem-based Microbiology. Problem-based basic science series. Saunders. p. 314. ISBN 9780721606309. Archived from the original on 24 April 2021. Retrieved 24 April 2021.
[Measles] virus habitat is humans.
- ^ "habitat". Dictionary.com Unabridged (Online). n.d.
- ^ "Habitat". Merriam-Webster Dictionary. Archived from the original on 26 December 2018. Retrieved 4 June 2016.
- ^ "Biotope". Oxford Dictionaries. Archived from the original on 4 August 2016. Retrieved 4 June 2016.
- ^ Everyman's Encyclopedia; Volume 4. J.M. Dent. 1967. p. 581. ASIN B0015GRC04.
- ^ Richards, O.W. (1940). "The biology of the small white butterfly (Pieris rapae), with special reference to the factors controlling its abundance". Journal of Animal Ecology. 9 (2): 243–288. Bibcode:1940JAnEc...9..243R. doi:10.2307/1459. JSTOR 1459.
- ^ Spitzer, L.; Benes, J.; Dandova, J.; Jaskova, V.; Konvicka, M. (2009). "The Large Blue butterfly (Phengaris [Maculinea] arion), as a conservation umbrella on a landscape scale: The case of the Czech Carpathians". Ecological Indicators. 9 (6): 1056–1063. Bibcode:2009EcInd...9.1056S. doi:10.1016/j.ecolind.2008.12.006.
- ^ Sutherland, William J.; Hill, David A. (1995). Managing habitat types for Conservation. Cambridge University Press. p. 6. ISBN 978-0-521-44776-8. Archived from the original on 12 December 2018. Retrieved 24 May 2016.
- ^ Richard J. Huggett (2004). Fundamentals of Biogeography. Psychology Press. p. 146. ISBN 978-0-415-32347-5. Archived from the original on 12 December 2018. Retrieved 24 May 2016.
- ^ "Invasive species". National Wildlife Federation. Archived from the original on 31 May 2016. Retrieved 24 May 2016.
- ^ Breed, Michael D.; Moore, Janice (2011). Animal Behavior. Academic Press. p. 248. ISBN 978-0-08-091992-8. Archived from the original on 12 December 2018. Retrieved 2 June 2016.
- ^ "Couch's spadefoot (Scaphiopus couchi)". Arizona–Sonora Desert Museum. Archived from the original on 30 May 2016. Retrieved 16 May 2016.
- ^ Witham, Carol W. (1998). Ecology, Conservation, and Management of Vernal Pool Ecosystems. California Native Plant Society. p. 1. ISBN 978-0-943460-37-6.
- ^ Green, Scott. "Fairy shrimp". The Vernal Pool Association. Archived from the original on 23 April 2016. Retrieved 17 May 2016.
- ^ Walker, Matt (21 May 2015). "The most extreme fish on Earth". BBC Earth. Archived from the original on 26 August 2016. Retrieved 17 May 2016.
- ^ a b "Habitats". BBC Nature. Archived from the original on 4 July 2016.
- ^ "UK Terrestrial & Freshwater Habitat Types: Freshwater Habitat descriptions" (PDF). Joint Nature Conservation Committee. Retrieved 16 May 2024.
- ^ Cook, C.D.K.; Gut, B.J.; Rix, E.M.; Schneller, J. (1974). Water Plants of the World: A Manual for the Identification of the Genera of Freshwater Macrophytes. Springer Science & Business Media. p. 7. ISBN 978-90-6193-024-2. Archived from the original on 12 December 2018. Retrieved 2 June 2016.
- ^ Roff, John (2013). Marine Conservation Ecology. Routledge. p. 105. ISBN 978-1-136-53838-4. Archived from the original on 12 December 2018. Retrieved 2 June 2016.
- ^ Read, Nicholas (2012). City Critters: Wildlife in the Urban Jungle. Orca Book Publishers. p. 2. ISBN 978-1-55469-394-8.
- ^ John G. Kelcey, John G. (2015). Vertebrates and Invertebrates of European Cities:Selected Non-Avian Fauna. Springer. p. 124. ISBN 978-1-4939-1698-6. Archived from the original on 12 December 2018. Retrieved 10 July 2016.
- ^ Abe, Y.; Bignell, David Edward; Higashi, T. (2014). Termites: Evolution, Sociality, Symbioses, Ecology. Springer. p. 437. ISBN 978-94-017-3223-9. Archived from the original on 12 December 2018. Retrieved 10 July 2016.
- ^ "Microhabitat types". Australian National Botanic Gardens, Centre for Australian National Biodiversity Research (An Australian Government Initiative). Government of the Commonwealth of Australia. Archived from the original on 14 April 2016. Retrieved 18 May 2016.
- ^ a b "Woodlands & Biodiversity". Offwell Woodland & Wildlife Trust. Archived from the original on 8 June 2016. Retrieved 18 May 2016.
- ^ Lewis, E.E.; Campbell, J.F.; Sukhdeo, M.V.K. (2002). The Behavioural Ecology of Parasites. CABI. p. 183. ISBN 978-0-85199-754-4. Archived from the original on 12 December 2018. Retrieved 10 July 2016.
- ^ Pung, Oscar J.; Burger, Ashley R.; Walker, Michael F.; Barfield, Whitney L.; Lancaster, Micah H.; Jarrous, Christina E. (2009). "In vitro cultivation of Microphallus turgidus (Trematoda: Microphallidae) from metacercaria to ovigerous adult with continuation of the life cycle in the laboratory". Journal of Parasitology. 95 (4): 913–919. doi:10.1645/ge-1970.1. JSTOR 27735680. PMID 20049996. S2CID 207250475.
- ^ Gorman, James (6 February 2013). "Bacteria Found Deep Under Antarctic Ice, Scientists Say". The New York Times. Archived from the original on 3 September 2019. Retrieved 18 May 2016.
- ^ Choi, Charles Q. (17 March 2013). "Microbes Thrive in Deepest Spot on Earth". LiveScience. Archived from the original on 2 April 2013. Retrieved 18 May 2016.
- ^ Oskin, Becky (14 March 2013). "Intraterrestrials: Life Thrives in Ocean Floor". LiveScience. Archived from the original on 2 April 2013. Retrieved 18 May 2016.
- ^ Schultz, Steven (13 December 1999). "Two miles underground". Princeton Weekly Bulletin. Archived from the original on 13 January 2016.
- ^ Chang, Kenneth (12 September 2016). "Visions of Life on Mars in Earth's Depths". New York Times. Archived from the original on 12 September 2016. Retrieved 12 September 2016.
- ^ Cole, Bob (March 2008). "Appendix 6". The SAA BUhlmann DeeP-Stop System Handbook. Sub-Aqua Association. pp. vi–1. ISBN 978-0-9532904-8-2.
- ^ a b "The Deep Sea". MarineBio Conservation Society. 29 December 2011. Archived from the original on 14 July 2018. Retrieved 19 May 2016.
- ^ "What does it take to live at the bottom of the ocean?". BBC Earth. 2016. Archived from the original on 13 May 2016. Retrieved 19 May 2016.
- ^ a b "A hydrothermal vent forms when seawater meets hot magma". Ocean facts. National Ocean Service. 11 January 2013. Archived from the original on 29 May 2016. Retrieved 20 May 2016.
- ^ "Hydrothermal Vent Creatures". Ocean Portal. Smithsonian National Museum of Natural History. Archived from the original on 24 May 2016. Retrieved 20 May 2016.
- ^ Desbruyères, Daniel; Segonzac, Michel (1997). Handbook of Deep-sea Hydrothermal Vent Fauna. Editions Quae. p. 9. ISBN 978-2-905434-78-4. Archived from the original on 12 December 2018. Retrieved 24 May 2016.
- ^ Womack, Ann M.; Bohannan, Brendan J.M.; Green, Jessica L. (2010). "Biodiversity and biogeography of the atmosphere". Philosophical Transactions of the Royal Society B. 365 (1558): 3645–3653. doi:10.1098/rstb.2010.0283. PMC 2982008. PMID 20980313.
- ^ Schulze-Makuch, Dirk; Haque, Shirin; Resendes de Sousa Antonio, Marina; Ali, Denzil; Hosein, Riad; Song, Young C.; Yang, Jinshu; Zaikova, Elena; Beckles, Denise M.; Guinan, Edward; Lehto, Harry J.; Hallam, Steven J. (2011). "Microbial Life in a Liquid Asphalt Desert". Astrobiology. 11 (3): 241–258. arXiv:1004.2047. Bibcode:2011AsBio..11..241S. doi:10.1089/ast.2010.0488. PMID 21480792. S2CID 22078593.
- ^ "Petroleum fly". Grzimek's Animal Life Encyclopedia. Vol. 3: Insects (2nd ed.). The Gale Group. 2004. p. 367. ISBN 978-0-7876-5779-6.
- ^ McGregor, G.B.; Rasmussen, J.P. (2008). "Cyanobacterial composition of microbial mats from an Australian thermal spring: a polyphasic evaluation". FEMS Microbiology Ecology. 63 (1): 23–35. Bibcode:2008FEMME..63...23M. doi:10.1111/j.1574-6941.2007.00405.x. PMID 18081588.
- ^ Hsing, Pen-Yuan (18 October 2010). "Gas-powered Circle of Life: Succession in a Deep-sea Ecosystem". Lophelia II 2010. NOAA. Archived from the original on 25 February 2014. Retrieved 22 May 2016.
- ^ a b Gostincar, C.; Grube, M.; De Hoog, S.; Zalar, P.; Gunde-Cimerman, N. (2010). "Extremotolerance in fungi: Evolution on the edge". FEMS Microbiology Ecology. 71 (1): 2–11. Bibcode:2010FEMME..71....2G. doi:10.1111/j.1574-6941.2009.00794.x. PMID 19878320.
- ^ Oren, Aharon (15 April 2008). "Microbial life at high salt concentrations: phylogenetic and metabolic diversity". Saline Systems. 4: 2. doi:10.1186/1746-1448-4-2. ISSN 1746-1448. PMC 2329653. PMID 18412960.
- ^ Perini, L.; Gostinčar, C.; Gunde-Cimerman, N. (27 December 2019). "Fungal and bacterial diversity of Svalbard subglacial ice". Scientific Reports. 9 (1): 20230. Bibcode:2019NatSR...920230P. doi:10.1038/s41598-019-56290-5. ISSN 2045-2322. PMC 6934841. PMID 31882659.
- ^ Takeuchi, Nozomu (2014). "Snow algae on Alaskan glaciers". Archived from the original on 29 March 2018. Retrieved 22 May 2016.
- ^ Lindenmayer, David B.; Fischer, Joern (2013). Habitat Fragmentation and Landscape Change: An Ecological and Conservation Synthesis. Island Press. pp. 1–10. ISBN 978-1-59726-606-2. Archived from the original on 12 December 2018. Retrieved 24 May 2016.
- ^ a b c Miller, G. Tyler; Spoolman, Scott (2008). Living in the Environment: Principles, Connections, and Solutions. Cengage Learning. pp. 193–195. ISBN 978-0-495-55671-8. Archived from the original on 12 December 2018. Retrieved 24 May 2016.
- ^ Holland, Matthew D.; Hastings, Alan (2008). "Strong effect of dispersal network structure on ecological dynamics". Nature. 456 (7223): 792–794. Bibcode:2008Natur.456..792H. doi:10.1038/nature07395. PMID 18931656. S2CID 4349469.
- ^ Brooks, M.L.; D'Antonio, C.M.; Richardson, D.M.; Grace, J.B.; Keeley, J.E.; DiTomaso, J.M.; Hobbs, R.J.; Pellant, M.; Pyke, D. (2004). "Effects of invasive alien plants on fire". BioScience. 54 (7): 677–688. doi:10.1641/0006-3568(2004)054[0677:EOIAPO]2.0.CO;2.
- ^ Lawrence, John M. (2013). Sea Urchins: Biology and Ecology. Academic Press. pp. 196–202. ISBN 978-0-12-397213-2. Archived from the original on 12 December 2018. Retrieved 10 July 2016.
- ^ "GLOBIO: Africa". GLOBIO. Archived from the original on 30 October 2005.
- ^ Schlaepfer, Daniel R.; Braschler, Brigitte; Rusterholz, Hans-Peter; Baur, Bruno (October 2018). "Genetic effects of anthropogenic habitat fragmentation on remnant animal and plant populations: a meta-analysis". Ecosphere. 9 (10). Bibcode:2018Ecosp...9E2488S. doi:10.1002/ecs2.2488. ISSN 2150-8925.
- ^ a b Sahney, S.; Benton, M. J.; Falcon-Lang, H. J. (1 December 2010). "Rainforest collapse triggered Pennsylvanian tetrapod diversification in Euramerica" (PDF). Geology. 38 (12): 1079–1082. Bibcode:2010Geo....38.1079S. doi:10.1130/G31182.1.
- ^ Fahrig, Lenore (2019). "Habitat fragmentation: A long and tangled tale". Global Ecology and Biogeography. 28 (1): 33–41. Bibcode:2019GloEB..28...33F. doi:10.1111/geb.12839. ISSN 1466-8238. S2CID 91260144.
- ^ Fahrig, L (2003). "Effects of habitat fragmentation on biodiversity". Annual Review of Ecology, Evolution, and Systematics. 34: 487–515. doi:10.1146/annurev.ecolsys.34.011802.132419.
- ^ Calizza, Edoardo; Costantini, Maria Letizia; Careddu, Giulio; Rossi, Loreto (17 June 2017). "Effect of habitat degradation on competition, carrying capacity, and species assemblage stability". Ecology and Evolution. 7 (15). Wiley: 5784–5796. Bibcode:2017EcoEv...7.5784C. doi:10.1002/ece3.2977. ISSN 2045-7758. PMC 5552933. PMID 28811883.
- ^ a b Sahney, S; Benton, Michael J.; Falcon-Lang, Howard J. (1 December 2010). "Rainforest collapse triggered Pennsylvanian tetrapod diversification in Euramerica" (PDF). Geology. 38 (12): 1079–1082. Bibcode:2010Geo....38.1079S. doi:10.1130/G31182.1. Archived from the original on 11 October 2011. Retrieved 29 November 2010 – via GeoScienceWorld.
- ^ Marvier, Michelle; Kareiva, Peter; Neubert, Michael G. (2004). "Habitat Destruction, Fragmentation, and Disturbance Promote Invasion by Habitat Generalists in a Multispecies Metapopulation". Risk Analysis. 24 (4): 869–878. Bibcode:2004RiskA..24..869M. doi:10.1111/j.0272-4332.2004.00485.x. ISSN 0272-4332. PMID 15357806. S2CID 44809930. Archived from the original on 23 July 2021. Retrieved 18 March 2021.
- ^ WIEGAND, THORSTEN; REVILLA, ELOY; MOLONEY, KIRK A. (February 2005). "Effects of Habitat Loss and Fragmentation on Population Dynamics". Conservation Biology. 19 (1): 108–121. Bibcode:2005ConBi..19..108W. doi:10.1111/j.1523-1739.2005.00208.x. ISSN 0888-8892. S2CID 33258495.
- ^ de Klemm, Cyrille (1997). Comparative Analysis of the Effectiveness of Legislation for the Protection of Wild Flora in Europe. Council of Europe. pp. 65–70. ISBN 978-92-871-3429-5. Archived from the original on 12 December 2018. Retrieved 10 July 2016.
- ^ "Convention on the Conservation of Migratory Species of Wild Animals". UNEP/CMS Secretariat. Archived from the original on 7 March 2011. Retrieved 7 July 2016.
- ^ Endangered Wildlife and Plants of the World. Marshall Cavendish. 2001. p. 750. ISBN 978-0-7614-7200-1. Archived from the original on 12 December 2018. Retrieved 10 July 2016.
- ^ Honey, Martha (2008). Ecotourism and Sustainable Development: Who Owns Paradise?. Island Press. p. 33. ISBN 978-1-59726-125-8.
- ^ Theel, Heather J.; Dibble, Eric D.; Madsen, John D. (2008). "Differential influence of a monotypic and diverse native aquatic plant bed on a macroinvertebrate assemblage; an experimental implication of exotic plant induced habitat". Hydrobiologia. 600: 77–87. doi:10.1007/s10750-007-9177-z. S2CID 19880476.
- ^ "1970 distribution of yellow starthistle in the U.S." Yellow Starthistle Information. UCD. Archived from the original on 31 December 2006.
- ^ "Invasive Mussels". National Wildlife Federation. Archived from the original on 17 August 2016. Retrieved 29 June 2016.

