Rolapitant
 | |
| Clinical data | |
|---|---|
| Pronunciation | /roʊˈlæpɪtænt/ roh-LAP-i-tant |
| Trade names | Varubi (US), Varuby (EU) |
| Other names | SCH 619734 |
| AHFS/Drugs.com | Monograph |
| MedlinePlus | a615041 |
| License data |
|
| Routes of administration | By mouth (tablets), intravenous |
| Drug class | NK1 receptor antagonists, antiemetics |
| ATC code | |
| Legal status | |
| Legal status | |
| Pharmacokinetic data | |
| Bioavailability | nearly 100% |
| Protein binding | 99.8% |
| Metabolism | CYP3A4 |
| Metabolites | C4-pyrrolidine-hydroxylated rolapitant (major) |
| Elimination half-life | 169–183 hours |
| Excretion | Feces (52–89%), urine (9–20%)[1] |
| Identifiers | |
| |
| CAS Number | |
| PubChem CID | |
| IUPHAR/BPS | |
| DrugBank | |
| ChemSpider | |
| UNII | |
| KEGG | |
| ChEBI | |
| CompTox Dashboard (EPA) | |
| ECHA InfoCard | 100.243.022 |
| Chemical and physical data | |
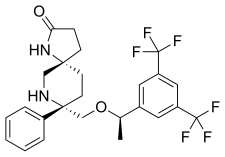
| Formula | C25H26F6N2O2 |
| Molar mass | 500.485 g·mol−1 |
| 3D model (JSmol) | |
| |
| |
Rolapitant (INN,[2] trade name Varubi /vəˈruːbi/ və-ROO-bee in the US and Varuby in the European Union) is a drug originally developed by Schering-Plough and licensed for clinical development by Tesaro, which acts as a selective NK1 receptor antagonist (antagonist for the NK1 receptor).[3] It has been approved as a medication for the treatment of chemotherapy-induced nausea and vomiting (CINV) after clinical trials showed it to have similar or improved efficacy and some improvement in safety over existing drugs for this application.[4][5][6][7]
Medical uses
[edit]Rolapitant is used in combination with other antiemetic (anti-vomiting) agents in adults for the prevention of delayed nausea and vomiting associated with initial and repeat courses of emetogenic cancer chemotherapy, including, but not limited to, highly emetogenic chemotherapy.[1] The approved antiemetic combination consists of rolapitant plus dexamethasone and a 5-HT3 antagonist.[8]
Contraindications
[edit]Under the US approval, rolapitant is contraindicated in combination with thioridazine, whose inactivation could be inhibited by rolapitant.[1] Under the European approval, it is contraindicated in combination with St. John's Wort, which is expected to accelerate inactivation of rolapitant.[8]
Side effects
[edit]In studies comparing chemotherapy plus rolapitant, dexamethasone and a 5-HT3 antagonist to chemotherapy plus placebo, dexamethasone and a 5-HT3 antagonist, most side effects had comparable frequencies in both groups, and differed more between chemotherapy regimens than between rolapitant and placebo groups. Common side effects included decreased appetite (9% under rolapitant vs. 7% under placebo), neutropenia (9% vs. 8% or 7% vs. 6%, depending on the kind of chemotherapy), dizziness (6% vs. 4%), indigestion and stomatitis (both 4% vs. 2%).[1]
Overdose
[edit]Up to eightfold therapeutic doses have been given in studies without problems.[8]
Interactions
[edit]Rolapitant moderately inhibits the liver enzyme CYP2D6. Blood plasma concentrations of the CYP2D6 substrate dextromethorphan have increased threefold when combined with rolapitant; and increased concentrations of other substrates are expected. The drug also inhibits the transporter proteins ABCG2 (breast cancer resistance protein, BCRP) and P-glycoprotein (P-gp), which has been shown to increase plasma concentrations of the ABCG2 substrate sulfasalazine twofold and the P-gp substrate digoxin by 70%.[8]
Strong inducers of the liver enzyme CYP3A4 decrease the area under the curve of rolapitant and its active metabolite (called M19); for rifampicin, this effect was almost 90% in a study. Inhibitors of CYP3A4 have no relevant effect on rolapitant concentrations.[8]
Pharmacology
[edit]Pharmacodynamics
[edit]Both rolapitant and its active metabolite M19 block the NK1 receptor with high affinity and selectivity: to block the closely related receptor NK2 or any other of 115 tested receptors and enzymes, more than 1000-fold therapeutic concentrations are necessary.[9]
Pharmacokinetics
[edit]
Rolapitant is practically completely absorbed from the gut, independently of food intake. It undergoes no measurable first-pass effect in the liver. Highest blood plasma concentrations are reached after about four hours. When in the bloodstream, 99.8% of the substance are bound to plasma proteins.[8]
It is metabolized by the liver enzyme CYP3A4, resulting in the major active metabolite M19 (C4-pyrrolidine-hydroxylated rolapitant) and a number of inactive metabolites. Rolapitant is mainly excreted via the feces (52–89%) in unchanged form, and to a lesser extent via the urine (9–20%) in form of its inactive metabolites. Elimination half-life is about seven days (169 to 183 hours) over a wide dosing range.[8]
Chemistry
[edit]The drug is used in form of rolapitant hydrochloride monohydrate, a white to off-white, slightly hygroscopic crystalline powder. Its maximum solubility in aqueous solutions is at pH 2–4.[9]
References
[edit]- ^ a b c d e "Varubi- rolapitant tablet". DailyMed. 6 August 2019. Retrieved 21 August 2020.
- ^ "International Nonproprietary Names for Pharmaceutical Substances (INN). Recommended International Nonproprietary Names (Rec. INN): List 59" (PDF). World Health Organization. p. 64. Retrieved 5 October 2016.
- ^ Duffy RA, Morgan C, Naylor R, Higgins GA, Varty GB, Lachowicz JE, Parker EM (July 2012). "Rolapitant (SCH 619734): a potent, selective and orally active neurokinin NK1 receptor antagonist with centrally-mediated antiemetic effects in ferrets". Pharmacology, Biochemistry, and Behavior. 102 (1): 95–100. doi:10.1016/j.pbb.2012.03.021. PMID 22497992. S2CID 24357198.
- ^ Jordan K, Jahn F, Aapro M (June 2015). "Recent developments in the prevention of chemotherapy-induced nausea and vomiting (CINV): a comprehensive review". Annals of Oncology. 26 (6): 1081–90. doi:10.1093/annonc/mdv138. PMID 25755107.
- ^ Nasir SS, Schwartzberg LS (August 2016). "Recent Advances in Preventing Chemotherapy-Induced Nausea and Vomiting". Oncology. 30 (8): 750–62. PMID 27539626.
- ^ Rapoport B, Schwartzberg L, Chasen M, Powers D, Arora S, Navari R, Schnadig I (April 2016). "Efficacy and safety of rolapitant for prevention of chemotherapy-induced nausea and vomiting over multiple cycles of moderately or highly emetogenic chemotherapy". European Journal of Cancer. 57: 23–30. doi:10.1016/j.ejca.2015.12.023. PMID 26851398.
- ^ Chasen MR, Rapoport BL (March 2016). "Rolapitant for the treatment of chemotherapy-induced nausea and vomiting: a review of the clinical evidence". Future Oncology. 12 (6): 763–78. doi:10.2217/fon.16.11. PMID 26842387.
- ^ a b c d e f g h "Varuby: EPAR – Product Information" (PDF). European Medicines Agency. 2017-05-31. Archived from the original (PDF) on 2018-03-18. Retrieved 2017-10-11.
- ^ a b "Varuby: EPAR – Public assessment report" (PDF). European Medicines Agency. 2017-05-31. Archived from the original (PDF) on 2018-03-18. Retrieved 2017-10-11.
External links
[edit]- "Rolapitant Injection: MedlinePlus Drug Information". MedlinePlus. 20 August 2020.
