R bodies
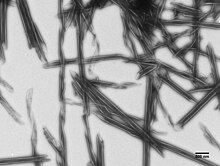
R bodies (from refractile bodies, also R-bodies) are polymeric protein inclusions formed inside the cytoplasm of bacteria.[1] Initially discovered in kappa particles, bacterial endosymbionts of the ciliate Paramecium, R bodies (and genes encoding them) have since been discovered in a variety of taxa.[2]
Morphology, assembly, and extension
[edit]At neutral pH, type 51 R bodies resemble a coil of ribbon approximately 500 nm in diameter and approximately 400 nm deep.[1] Encoded by a single operon containing four open reading frames,[3][4] R bodies are formed from two small structural proteins, RebA and RebB.[5] A third protein, RebC, is required for the covalent assembly of these two structural proteins into higher-molecular weight products, visualized as a ladder on an SDS-PAGE gel.[5]
At low pH, Type 51 R bodies undergo a dramatic structural rearrangement. Much like a paper yo-yo, the ribbon extends (from the center) to form hollow tube with pointed ends that can reach up to 20μm in length.[6]
Other types of R bodies from different bacterial species vary in their size, ribbon morphology, and triggers for extension.[1]
Function
[edit]When kappa particles shed from a killer paramecium are ingested, R bodies extend within the acidic food vacuole of the predatory paramecium, distending and rupturing the membrane.[7] This liberates the contents of the food vacuole into the cytoplasm of the paramecium.[7] While feeding kappa particles to sensitive paramecium results in the death of paramecium, feeding purified R bodies or R bodies recombinantly expressed in E. coli is not toxic.[3][8] Thus, R bodies are thought to function as a toxin delivery system.
R bodies are also capable of rupturing E. coli spheroplasts, demonstrating that they can rupture membranes in a foreign context, and they can be engineered to extend at a variety of different pH levels.[9]
References
[edit]- ^ a b c Pond, F R; Gibson, I; Lalucat, J; Quackenbush, R L (1989-03-01). "R-body-producing bacteria". Microbiological Reviews. 53 (1): 25–67. doi:10.1128/mr.53.1.25-67.1989. ISSN 0146-0749. PMC 372716. PMID 2651865.
- ^ Raymann, Kasie; Bobay, Louis-Marie; Doak, Thomas G.; Lynch, Michael; Gribaldo, Simonetta (2013-03-01). "A genomic survey of Reb homologs suggests widespread occurrence of R-bodies in proteobacteria". G3: Genes, Genomes, Genetics. 3 (3): 505–516. doi:10.1534/g3.112.005231. ISSN 2160-1836. PMC 3583457. PMID 23450193.
- ^ a b Kanabrocki, J. A.; Quackenbush, R. L.; Pond, F. R. (1986-10-01). "Organization and expression of genetic determinants for synthesis and assembly of type 51 R bodies". Journal of Bacteriology. 168 (1): 40–48. doi:10.1128/jb.168.1.40-48.1986. ISSN 0021-9193. PMC 213417. PMID 3759909.
- ^ Jeblick, Jörn; Kusch, Jürgen (2005-02-01). "Sequence, transcription activity, and evolutionary origin of the R-body coding plasmid pKAP298 from the intracellular parasitic bacterium Caedibacter taeniospiralis". Journal of Molecular Evolution. 60 (2): 164–173. doi:10.1007/s00239-004-0002-2. ISSN 0022-2844. PMID 15785846. S2CID 6301794.
- ^ a b Heruth, D. P.; Pond, F. R.; Dilts, J. A.; Quackenbush, R. L. (1994-06-01). "Characterization of genetic determinants for R body synthesis and assembly in Caedibacter taeniospiralis 47 and 116". Journal of Bacteriology. 176 (12): 3559–3567. doi:10.1128/jb.176.12.3559-3567.1994. ISSN 0021-9193. PMC 205544. PMID 8206833.
- ^ Preer, John R.; Hufnagel, Linda A.; Preer, Louise B. (1966-04-01). "Structure and behavior of R bodies from killer paramecia". Journal of Ultrastructure Research. 15 (1): 131–143. doi:10.1016/S0022-5320(66)80100-4. PMID 5936490.
- ^ a b Mueller, Jo Anne (1965-12-01). "Vitally stained kappa in Paramecium aurelia". Journal of Experimental Zoology. 160 (3): 369–372. doi:10.1002/jez.1401600314. ISSN 1097-010X. PMID 4160786.
- ^ Schrallhammer, Martina; Galati, Stefano; Altenbuchner, Josef; Schweikert, Michael; Görtz, Hans-Dieter; Petroni, Giulio (2012-11-01). "Tracing the role of R-bodies in the killer trait: absence of toxicity of R-body producing recombinant E. coli on paramecia". European Journal of Protistology. 48 (4): 290–296. doi:10.1016/j.ejop.2012.01.008. ISSN 1618-0429. PMID 22356923.
- ^ Polka, Jessica K.; Silver, Pamela A. (2016-04-15). "A Tunable Protein Piston That Breaks Membranes to Release Encapsulated Cargo" (PDF). ACS Synthetic Biology. 5 (4): 303–311. doi:10.1021/acssynbio.5b00237. PMID 26814170.
