Protic ionic liquid
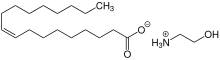
A protic ionic liquid is an ionic liquid that is formed via proton transfer from a Brønsted acid to a Brønsted base.[1] Unlike many other types of ionic liquids, which are formed through a series of synthesis steps,[2] protic ionic liquids are easier to create because the acid and base must simply be mixed together.[1]
Properties
[edit]Because the proton transfer reaction is reversible, the equilibrium between reactants and ionic products can shift depending on the conditions. This equilibration significantly impact the properties of protic ionic liquids since some neutral acid and base species are generally present in the solution.
Many protic ionic liquids have non-negligible vapor pressure,[1] even though traditional ionic liquids are often touted for their low vapor pressures.[3][4] Some protic ionic liquids are distillable.[1][5][6] Additionally, during distillation protic ionic liquids exhibit what appears to be a reactive azeotrope.[7][8][6] In other words, initially only the acid or base is vaporized, but at a specific composition both the acid and base start to vaporize and the composition of the vapor and liquid is the same. This composition depends on the anion and cation and contains more acid than base.[7] However, when mixed with water the vaporization behavior is different and an azeotrope no longer occurs at that same composition.[9]
The density of a protic ionic liquid mixture also appears to be higher for the composition at which the azeotrope forms. That is, if some excess of the acid is present in the mixture the density increases.[10]
References
[edit]- ^ a b c d Greaves, Tamar L.; Drummond, Calum J. (2008-01-01). "Protic Ionic Liquids: Properties and Applications". Chemical Reviews. 108 (1): 206–237. doi:10.1021/cr068040u. ISSN 0009-2665. PMID 18095716.
- ^ Ratti, Rajni (2014-10-29). "Ionic Liquids: Synthesis and Applications in Catalysis". Advances in Chemistry. 2014: 1–16. doi:10.1155/2014/729842.
- ^ Rogers, Robin D.; Seddon, Kenneth R. (2003-10-31). "Ionic Liquids--Solvents of the Future?". Science. 302 (5646): 792–793. doi:10.1126/science.1090313. ISSN 0036-8075. PMID 14593156. S2CID 94376105.
- ^ Earle, Martyn J.; Esperança, José M. S. S.; Gilea, Manuela A.; Canongia Lopes, José N.; Rebelo, Luís P. N.; Magee, Joseph W.; Seddon, Kenneth R.; Widegren, Jason A. (February 2006). "The distillation and volatility of ionic liquids". Nature. 439 (7078): 831–834. Bibcode:2006Natur.439..831E. doi:10.1038/nature04451. ISSN 1476-4687. PMID 16482154. S2CID 4357175.
- ^ Parviainen, Arno; King, Alistair W. T.; Mutikainen, Ilpo; Hummel, Michael; Selg, Christoph; Hauru, Lauri K. J.; Sixta, Herbert; Kilpeläinen, Ilkka (2013-11-01). "Predicting Cellulose Solvating Capabilities of Acid–Base Conjugate Ionic Liquids". ChemSusChem. 6 (11): 2161–2169. doi:10.1002/cssc.201300143. ISSN 1864-5631. PMID 24106149.
- ^ a b Ahmad, Waqar; Ostonen, Alexandr; Jakobsson, Kaj; Uusi-Kyyny, Petri; Alopaeus, Ville; Hyväkkö, Uula; King, Alistair W. T. (2016-10-01). "Feasibility of thermal separation in recycling of the distillable ionic liquid [DBNH][OAc] in cellulose fiber production". Chemical Engineering Research and Design. 114: 287–298. doi:10.1016/j.cherd.2016.08.032. ISSN 0263-8762.
- ^ a b Ribeiro, Filipe M. S.; Lima, Carlos F. R. A. C.; Silva, Artur M. S.; Santos, Luís M. N. B. F. (2018-09-18). "Experimental Evidence for Azeotrope Formation from Protic Ionic Liquids". ChemPhysChem. 19 (18): 2364–2369. doi:10.1002/cphc.201800335. ISSN 1439-4235. PMID 29799151. S2CID 44127885.
- ^ Lopes, José N. Canongia; Rebelo, Luís Paulo N. (2010-02-09). "Ionic liquids and reactive azeotropes: the continuity of the aprotic and protic classes". Physical Chemistry Chemical Physics. 12 (8): 1948–1952. Bibcode:2010PCCP...12.1948L. doi:10.1039/B922524M. ISSN 1463-9084. PMID 20145863.
- ^ Baird, Zachariah Steven; Uusi-Kyyny, Petri; Witos, Joanna; Rantamäki, Antti H.; Sixta, Herbert; Wiedmer, Susanne K.; Alopaeus, Ville (2020-05-14). "Vapor–Liquid Equilibrium of Ionic Liquid 7-Methyl-1,5,7-triazabicyclo[4.4.0]dec-5-enium Acetate and Its Mixtures with Water". Journal of Chemical & Engineering Data. 65 (5): 2405–2421. doi:10.1021/acs.jced.9b01039. ISSN 0021-9568.
- ^ Baird, Zachariah Steven; Uusi-Kyyny, Petri; Dahlberg, Artur; Cederkrantz, Daniel; Alopaeus, Ville (2020-10-01). "Densities, Viscosities, and Thermal Conductivities of the Ionic Liquid 7-Methyl-1,5,7-triazabicyclo[4.4.0]dec-5-enium Acetate and Its Mixtures with Water". International Journal of Thermophysics. 41 (12): 160. Bibcode:2020IJT....41..160B. doi:10.1007/s10765-020-02742-4. ISSN 1572-9567.
