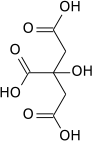
Propane-1,2,3-tricarboxylic acid

| |
| Names | |
|---|---|
| Preferred IUPAC name
Propane-1,2,3-tricarboxylic acid | |
Other names
| |
| Identifiers | |
3D model (JSmol)
|
|
| ChemSpider | |
| ECHA InfoCard | 100.002.485 |
PubChem CID
|
|
| UNII | |
CompTox Dashboard (EPA)
|
|
| |
| |
| Properties | |
| C6H8O6 | |
| Molar mass | 176.124 g·mol−1 |
| Melting point | 156-161 |
| Soluble | |
| Related compounds | |
Related compounds
|
citric acid |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
| |
Propane-1,2,3-tricarboxylic acid, also known as tricarballylic acid, carballylic acid, and β-carboxyglutaric acid, is a tricarboxylic acid. The compound is an inhibitor of the enzyme aconitase and therefore interferes with the Krebs cycle.[1]
Esters of propane-1,2,3-tricarboxylic acid are found in natural products such as the mycotoxins fumonisins B1 and B2 and AAL toxin TA, and in macrocyclic inhibitors of Ras farnesyl-protein transferase (FPTase) such as actinoplanic acid.
Propane-1,2,3-tricarboxylic acid can be synthesized in two steps from fumaric acid.[2]
Mechanism of the inhibition of aconitase
[edit]Aconitase normally catalyses, via the intermediate aconitic acid, the interconversion of citric acid into isocitric acid. Propane-1,2,3-tricarboxylic acid is well suited to bind to aconitase as it only lacks the hydroxyl group in comparison to citric acid. However, the hydroxyl group is essential to proceed from citric acid to aconitic acid; therefore, the enzyme is not able to complete the reaction with propane-1,2,3-tricarboxylic acid.
References
[edit]- ^ Russell, James B.; Forsberg, Neil (1986). "Production of tricarballylic acid by rumen microorganisms and its potential toxicity in ruminant tissue metabolism". British Journal of Nutrition. 56 (1): 153–62. doi:10.1079/BJN19860095. PMID 3676191.
- ^ H. T. Clarke; T. F. Murray (1941). "Tricarballylic Acid". Organic Syntheses; Collected Volumes, vol. 1, p. 523.